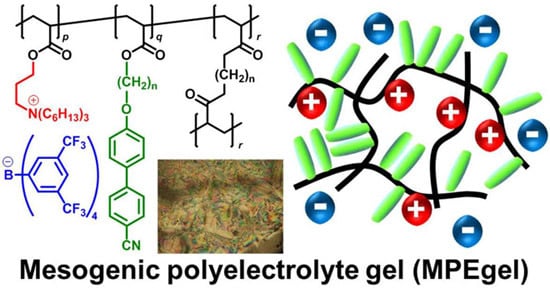
Mesogenic Polyelectrolyte Gels Absorb Organic Solvents and Liquid Crystalline Molecules
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Measurements
2.2. Synthesis of Compound 1
2.3. Synthesis of Monomer M5
2.4. Synthesis of Compound 2
2.5. Synthesis of Monomer M6
2.6. Preparation of MPEG5-p and MPEG6-p
2.7. Preparation of MPEP5-5
2.8. Swelling Degree Measurement
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MPE | Mesogenic Polyelectrolyte |
References
- Dautzenberg, H.; Jaeger, W.; Kötz, J.; Philipp, B.; Seidel, C.; Stscherbina, D. Polyelectrolytes; Carl Hanser Verlag: Munich, Germany, 1994. [Google Scholar]
- Marko, J.F.; Siggia, E.D. Stretching DNA. Macromolecules 1995, 28, 8759–8770. [Google Scholar] [CrossRef]
- Wong, G.C.L.; Pollack, L. Electrostatics of strongly charged biological polymers: Ion-mediated interactions and self-organization in nucleic acids and proteins. Annu. Rev. Phys. Chem. 2010, 61, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Hill, J.P.; Ji, Q. Layer-by-layer assembly as a versatile bottom-up nanofabrication technique for exploratory research and realistic application. Phys. Chem. Chem. Phys. 2007, 9, 2319–2340. [Google Scholar] [CrossRef] [PubMed]
- Rivas, B.L.; Pereira, E.D.; Moreno-Villoslada, I. Water-soluble polymer–metal ion interactions. Prog. Polym. Sci. 2003, 28, 173–208. [Google Scholar] [CrossRef]
- Fang, J.; Guo, X.; Harada, S.; Watari, T.; Tanaka, K.; Kita, H.; Okamoto, K.-I. Novel sulfonated polyimides as polyelectrolytes for fuel cell application. 1. Synthesis, proton conductivity, and water stability of polyimides from 4,4’-diaminodiphenyl ether-2,2’-disulfonic acid. Macromolecules 2002, 35, 9022–9028. [Google Scholar] [CrossRef]
- Xing, P.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Wang, K.; Kaliaguine, K.S. Synthesis and characterization of sulfonated poly(ether ether ketone) for proton exchange membranes. J. Membr. Sci. 2004, 229, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Asano, N.; Aoki, M.; Suzuki, S.; Miyatake, K.; Uchida, H.; Watanabe, M. Aliphatic/aromatic polyimide ionomers as a proton conductive membrane for fuel cell applications. J. Am. Chem. Soc. 2006, 128, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Lu, S.; Li, Y.; Huang, A.; Zhuang, L.; Lu, J. High-performance alkaline polymer electrolyte for fuel cell applications. Adv. Funct. Mater. 2010, 20, 312–319. [Google Scholar] [CrossRef]
- Wang, S.; Yu, D.; Dai, L. Polyelectrolyte functionalized carbon nanotubes as efficient metal-free electrocatalysts for oxygen reduction. J. Am. Chem. Soc. 2011, 133, 5182–5185. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T. Gels. Sci. Am. 1981, 244, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Osada, Y.; Ross-Murphy, S.B. Intelligent gels. Sci. Am. 1993, 268, 82–87. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H.; Dobrynin, A.V.; Joanny, J.-F. Elastic modulus and equilibrium swelling of polyelectrolyte gels. Macromolecules 1996, 29, 398–406. [Google Scholar] [CrossRef]
- Philippova, O.E.; Rulkens, R.; Kovtunenko, B.I.; Abramchuk, S.S.; Khokhlov, A.R.; Wegner, G. Polyacrylamide hydrogels with trapped polyelectrolyte rods. Macromolecules 1998, 31, 1168–1179. [Google Scholar] [CrossRef]
- Hong, W.; Zhao, X.; Suo, Z. Large deformation and electrochemistry of polyelectrolyte gels. J. Mech. Phys. Solids 2010, 58, 558–577. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Quesada-Pérez, M.; Maroto-Centeno, J.A.; Forcada, J.; Hidalgo-Alvarez, R. Gel swelling theories: The classical formalism and recent approaches. Soft Matter 2011, 7, 10536–10547. [Google Scholar] [CrossRef]
- Lvov, Y.; Decher, G.; Mohwald, H. Assembly, structural characterization, and thermal behavior of layer-by-layer deposited ultrathin films of poly(vinyl sulfate) and poly(allylamine). Langmuir 1993, 9, 481–486. [Google Scholar] [CrossRef]
- Mamedov, A.A.; Kotov, N.A.; Prato, M.; Guldi, D.M.; Wicksted, J.P.; Hirsch, A. Molecular design of strong single-wall carbon nanotube/polyelectrolyte multilayer composites. Nat. Mater. 2002, 1, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Picart, C.; Mutterer, J.; Richert, L.; Luo, Y.; Prestwich, G.D.; Schaaf, P.; Voegel, J.-C.; Lavalle, P. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc. Natl. Acad. Sci. USA 2002, 99, 12531–12535. [Google Scholar] [CrossRef] [PubMed]
- Peyratout, C.S.; Dähne, L. Tailor-made polyelectrolyte microcapsules: From multilayers to smart containers. Angew. Chem. Int. Ed. 2004, 43, 3762–3783. [Google Scholar] [CrossRef] [PubMed]
- Boudou, T.; Crouzier, T.; Ren, K.; Blin, G.; Picart, C. Multiple functionalities of polyelectrolyte multilayer films: new biomedical applications. Adv. Mater. 2010, 22, 441–467. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Lvov, Y.M.; Kawakami, K.; Ji, Q.; Hill, J.P. Layer-by-layer self-assembled shells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Lu, S.; Jiang, S.P. Layer-by-layer self-assembly in the development of electrochemical energy conversion and storage devices from fuel cells to supercapacitors. Chem. Soc. Rev. 2012, 41, 7291–7321. [Google Scholar] [CrossRef] [PubMed]
- Nomula, S.; Cooper, S.L. Ionomer solution structure and solution diagram. Macromolecules 2001, 34, 2653–2659. [Google Scholar] [CrossRef]
- Ono, T.; Ohta, M.; Sada, K. Ionic polymers act as polyelectrolytes in nonpolar media. ACS Macro Lett. 2012, 1, 1270–1273. [Google Scholar] [CrossRef]
- Ono, T.; Sugimoto, T.; Shinkai, S.; Sada, K. Lipophilic polyelectrolyte gels as super-absorbent polymers for nonpolar organic solvents. Nat. Mater. 2007, 6, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Shinkai, S.; Sada, K. Discontinuous swelling behaviors of lipophilic polyelectrolyte gels in non-polar media. Soft Matter 2008, 4, 748–750. [Google Scholar] [CrossRef]
- Ono, T.; Sugimoto, T.; Shinkai, S.; Sada, K. Molecular design of superabsorbent polymers for organic solvents by crosslinked lipophilic polyelectrolytes. Adv. Funct. Mater. 2008, 18, 3936–3940. [Google Scholar] [CrossRef]
- Iseda, K.; Ohta, M.; Ono, T.; Sada, K. High swelling ability of polystyrene-based polyelectrolyte gels at low temperature. Soft Matter 2011, 7, 5938–5940. [Google Scholar] [CrossRef]
- Ono, T.; Ohta, M.; Iseda, K.; Sada, K. Counter anion dependent swelling behaviour of poly(octadecyl acrylate)-based lipophilic polyelectrolyte gels as superabsorbent polymers for organic solvents. Soft Matter 2012, 8, 3700–3704. [Google Scholar] [CrossRef]
- Ono, T.; Sada, K. Toward the design of superabsorbent materials for non-polar organic solvents and oils: Ionic content dependent swelling behaviour of cross-linked poly(octadecyl acrylate)-based lipophilic polyelectrolytes. J. Mater. Chem. 2012, 22, 20962–20967. [Google Scholar] [CrossRef]
- Krishnamurthi, J.; Ono, T.; Amemori, S.; Komatsu, H.; Shinkai, S.; Sada, K. Thiourea-tagged poly(octadecyl acrylate) gels as fluoride and acetate responsive polymer gels through selective complexation. Chem. Commun. 2011, 47, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Iseda, K.; Haketa, Y.; Kokado, K.; Maeda, H.; Furuta, H.; Sada, K. Visualization of the complexation between chloride and anion receptors using volume change of ionomer gels in organic solvents. Soft Matter 2012, 8, 7490–7494. [Google Scholar] [CrossRef]
- Nishi, K.; Tochioka, S.; Hiroi, T.; Yamada, T.; Kokado, K.; Kim, T.-H.; Gilbert, E.P.; Sada, K.; Shibayama, M. Structural analysis of lipophilic polyelectrolyte solutions and gels in low-polar solvents. Macromolecules 2015, 48, 3613–3621. [Google Scholar] [CrossRef]
- Ohta, M.; Ono, T.; Sada, K. Layer-by-layer deposition of ionomers with lipophilic ion-pairs dissociated in less-polar media. Chem. Lett. 2011, 40, 648–650. [Google Scholar] [CrossRef]
- Yamada, T.; Kokado, K.; Higaki, Y.; Takahara, A.; Sada, K. Preparation and morphology variation of lipophilic polyelectrolyte brush functioning in nonpolar solvents. Chem. Lett. 2014, 43, 1300–1302. [Google Scholar] [CrossRef]
- Ohta, M.; Ono, T.; Kokado, K.; Kakugo, A.; Sada, K. Lipophilic ionomers with bulky ion-pairs and effect of counterion on miscibility of the ionomer blends. Macromol. Chem. Phys. 2016, 217, 433–444. [Google Scholar] [CrossRef]
- Chandrasekhar, S. Liquid Crystals, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Ichimura, K. Photoalignment of liquid-crystal systems. Chem. Rev. 2000, 100, 1847–1873. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.A. Liquid Gold: The Story of Liquid Crystal Displays and the Creation of an Industry; World Scientific Publishing: Singapore, 2005. [Google Scholar]
- Sabnis, R.W. Color filter technology for liquid crystal displays. Displays 1999, 20, 119–129. [Google Scholar] [CrossRef]
- Gray, G.W.; Kelly, S.M. Liquid crystals for twisted nematic display devices. J. Mater. Chem. 1999, 9, 2037–2050. [Google Scholar] [CrossRef]
- Kirsch, P.; Bremer, M. Nematic liquid crystals for active matrix displays: Molecular design and synthesis. Angew. Chem. Int. Ed. 2000, 39, 4216–4235. [Google Scholar] [CrossRef]
- Cao, W.; Muñoz, A.; Palffy-Muhoray, P.; Taheri, B. Lasing in a three-dimensional photonic crystal of the liquid crystal blue phase II. Nat. Mater. 2002, 1, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.; Kasano, M.; Ganzke, D.; Haase, W.; Yoshino, K. Mirrorless lasing in a dye-doped ferroelectric liquid crystal. Adv. Mater. 2002, 14, 306–309. [Google Scholar] [CrossRef]
- Coles, H.; Morris, S. Liquid-crystal lasers. Nat. Photonics 2010, 4, 676–685. [Google Scholar] [CrossRef]
- Sergeyev, S.; Pisula, W.; Geerts, Y.H. Discotic liquid crystals: A new generation of organic semiconductors. Chem. Soc. Rev. 2007, 36, 1902–1929. [Google Scholar] [CrossRef] [PubMed]
- Akagi, K.; Piao, G.; Kaneko, S.; Sakamaki, K.; Shirakawa, H.; Kyotani, M. Helical polyacetylene synthesized with a chiral nematic reaction field. Science 1998, 282, 1683–1686. [Google Scholar] [CrossRef] [PubMed]
- Akagi, K. Helical polyacetylene: Asymmetric polymerization in a chiral liquid-crystal field. Chem. Rev. 2009, 109, 5354–5401. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hirai, Y.; Nakaso, S.; Moriyama, M. Liquid-crystalline physical gels. Chem. Soc. Rev. 2007, 36, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Urayama, K. Selected issues in liquid crystal elastomers and gels. Macromolecules 2007, 40, 2277–2288. [Google Scholar] [CrossRef]
- Tsuchitani, A.; Ashida, H.; Urayama, K. Pronounced effects of cross-linker geometries on the orientation coupling between dangling mesogens and network backbones in side-chain type liquid crystal elastomers. Polymer 2015, 61, 29–35. [Google Scholar] [CrossRef]
- Tong, X.; Zhao, Y. Liquid-crystal gel-dispersed quantum dots: Reversible modulation of photoluminescence intensity using an electric field. J. Am. Chem. Soc. 2007, 129, 6372–6373. [Google Scholar] [CrossRef] [PubMed]
- Puech, N.; Blanc, C.; Grelet, E.; Zamora-Ledezma, C.; Maugey, M.; Zakri, C.; Anglaret, E.; Poulin, P. Highly ordered carbon nanotube nematic liquid Crystals. J. Phys. Chem. C 2011, 115, 3272–3278. [Google Scholar] [CrossRef]
- Blanc, C.; Coursault, D.; Lacaze, E. Ordering nano- and microparticles assemblies with liquid crystals. Liquid Crystals Rev. 2013, 1, 83–109. [Google Scholar] [CrossRef]
- Chena, F.-L.; Jamiesona, A.M. Odd–even effect in miscibility of main-chain liquid crystal polymers with low molar mass nematogens. Liquid Crystals 1993, 15, 171–183. [Google Scholar] [CrossRef]





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikori, Y.; Iseda, K.; Kokado, K.; Sada, K. Mesogenic Polyelectrolyte Gels Absorb Organic Solvents and Liquid Crystalline Molecules. Polymers 2016, 8, 148. https://doi.org/10.3390/polym8040148
Nishikori Y, Iseda K, Kokado K, Sada K. Mesogenic Polyelectrolyte Gels Absorb Organic Solvents and Liquid Crystalline Molecules. Polymers. 2016; 8(4):148. https://doi.org/10.3390/polym8040148
Chicago/Turabian StyleNishikori, Yusuke, Kazuya Iseda, Kenta Kokado, and Kazuki Sada. 2016. "Mesogenic Polyelectrolyte Gels Absorb Organic Solvents and Liquid Crystalline Molecules" Polymers 8, no. 4: 148. https://doi.org/10.3390/polym8040148
APA StyleNishikori, Y., Iseda, K., Kokado, K., & Sada, K. (2016). Mesogenic Polyelectrolyte Gels Absorb Organic Solvents and Liquid Crystalline Molecules. Polymers, 8(4), 148. https://doi.org/10.3390/polym8040148