Stereocomplex-Reinforced PEGylated Polylactide Micelle for Optimized Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
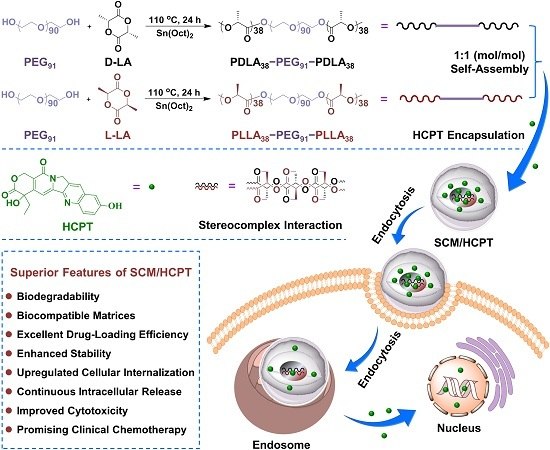
2.2. Syntheses of PDLA–PEG–PDLA and PLLA–PEG–PLLA Triblock Copolymers
2.3. Micelle Preparations
2.4. HCPT Encapsulations
2.5. Measurements
2.6. In Vitro HCPT Release
2.7. HCPT Uptake
2.8. Cytotoxicity Assays
3. Results and Discussion
3.1. Syntheses and Characterizations of PLA–PEG–PLA Triblock Copolymers
3.2. Self-Assembly Behaviors and Mechanism of Stereocomplex Interaction
3.3. In Vitro HCPT Loading and Release
3.4. Upregulated Cellular Uptake and Antitumor Activity of SCM/HCPT
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brzeziński, M.; Biela, T. Micro- and nanostructures of polylactide stereocomplexes and their biomedical applications. Polym. Int. 2015, 64, 1667–1675. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Jiang, D.; Wang, S.J.; Qi, Y.S.; Ding, J.X.; Yu, J.K.; Chen, X.S. Scaffolds drive meniscus tissue engineering. RSC Adv. 2015, 5, 77851–77859. [Google Scholar] [CrossRef]
- Li, X.; Ding, J.; Wang, J.; Zhuang, X.; Chen, X. Biomimetic biphasic scaffolds for osteochondral defect repair. Regen. Biomater. 2015, 2, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.H.; Ding, J.X.; Xu, W.G.; Song, X.F.; Zhuang, X.L.; Chen, X.S. Stereocomplex micelles based on 4-armed poly(ethylene glycol)-polylactide enantiomeric copolymers for drug delivery. Acta Polym. Sin. 2014, 9, 1265–1273. [Google Scholar]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K. Polylactide (PLA)-based amphiphilic block copolymers: Synthesis, self-assembly, and biomedical applications. Soft Matter 2011, 7, 5096–5108. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.Y.; Nieh, M.P.; Lai, P.S. Facile self-assembly of porphyrin-embedded polymeric vesicles for theranostic applications. Chem. Commun. 2012, 48, 9343–9345. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, K.; Xu, W.; Ding, J.; Wang, X.; Liu, T.; Wang, C.; Chen, X. Stereocomplex micelle from nonlinear enantiomeric copolymers efficiently transports antineoplastic drug. Nanoscale Res. Lett. 2015, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Chung, H.; Im, S.; Park, Y.; Kim, C.; Kim, S.B.; Rha, S.; Lee, M.; Ro, J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhuang, X.; Xiao, C.; Cheng, Y.; Zhao, L.; He, C.; Tang, Z.; Chen, X. Preparation of photo-cross-linked pH-responsive polypeptide nanogels as potential carriers for controlled drug delivery. J. Mater. Chem. 2011, 21, 11383–11391. [Google Scholar] [CrossRef]
- Samarajeewa, S.; Shrestha, R.; Elsabahy, M.; Karwa, A.; Li, A.; Zentay, R.P.; Kostelc, J.G.; Dorshow, R.B.; Wooley, K.L. In vitro efficacy of paclitaxel-loaded dual-responsive shell cross-linked polymer nanoparticles having orthogonally degradable disulfide cross-linked corona and polyester core domains. Mol. Pharm. 2013, 10, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chen, L.; Xiao, C.; Chen, L.; Zhuang, X.; Chen, X. Noncovalent interaction-assisted polymeric micelles for controlled drug delivery. Chem. Commun. 2014, 50, 11274–11290. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Pratt, R.C.; Nederberg, F.; Tan, J.P.; Yang, Y.Y.; Waymouth, R.M.; Hedrick, J.L. Organocatalytic approach to amphiphilic comb-block copolymers capable of stereocomplexation and self-assembly. Biomacromolecules 2008, 9, 3051–3056. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qi, X.; Liu, P.; El Ghzaoui, A.; Li, S. Aggregation behavior of self-assembling polylactide/poly(ethylene glycol) micelles for sustained drug delivery. Int. J. Pharm. 2010, 394, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Ding, J.; Lu, S.; Wang, X.; Wang, C.; Chen, X. Cholesterol-enhanced polylactide-based stereocomplex micelle for effective delivery of doxorubicin. Materials 2015, 8, 216–230. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Z.; Hu, J.; Chen, X.; Jing, X. Enantiomeric PLA–PEG block copolymers and their stereocomplex micelles used as rifampin delivery. J. Nanopart. Res. 2007, 9, 777–785. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Z.; Chen, L.; Cao, Y.; He, C.; Chen, X. Biodegradable stereocomplex micelles based on dextran-block-polylactide as efficient drug deliveries. Langmuir 2013, 29, 13072–13080. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Shi, F.; Li, D.; Chen, L.; Zhuang, X.; Chen, X. Enhanced endocytosis of acid-sensitive doxorubicin derivatives with intelligent nanogel for improved security and efficacy. Biomater. Sci. 2013, 1, 633–646. [Google Scholar] [CrossRef]
- Li, D.; Ding, J.X.; Tang, Z.H.; Sun, H.; Zhuang, X.L.; Xu, J.Z.; Chen, X.S. In vitro evaluation of anticancer nanomedicines based on doxorubicin and amphiphilic Y-shaped copolymers. Int. J. Nanomed. 2012, 7, 2687–2697. [Google Scholar]
- Sánchez-Iglesias, A.; Grzelczak, M.; Altantzis, T.; Goris, B.; Pérez-Juste, J.; Bals, S.; van Tendeloo, G.; Donaldson, S.H.; Chmelka, B.F.; Israelachvili, J.N.; et al. Hydrophobic interactions modulate self-assembly of nanoparticles. ACS Nano 2012, 6, 11059–11065. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chen, J.; Li, D.; Xiao, C.; Zhang, J.; He, C.; Zhuang, X.; Chen, X. Biocompatible reduction-responsive polypeptide micelles as nanocarriers for enhanced chemotherapy efficacy in vitro. J. Mater. Chem. B 2013, 1, 69–81. [Google Scholar] [CrossRef]
- Kang, N.; Perron, M.-È.; Prud’Homme, R.E.; Zhang, Y.; Gaucher, G.; Leroux, J.C. Stereocomplex block copolymer micelles: Core-shell nanostructures with enhanced stability. Nano Lett. 2005, 5, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Xu, W.; Zhang, Y.; Sun, D.; Xiao, C.; Liu, D.; Zhu, X.; Chen, X. Self-reinforced endocytoses of smart polypeptide nanogels for “on-demand” drug delivery. J. Control. Release 2013, 172, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; What is the appropriate target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Pan, P.; Shan, G.; Bao, Y.; Fujita, M.; Maeda, M. Core-shell structure, biodegradation, and drug release behavior of poly(lactic acid)/poly(ethylene glycol) block copolymer micelles tuned by macromolecular stereostructure. Langmuir 2015, 31, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yue, Z.G.; Qu, J.B.; Yue, H.; Su, Z.G.; Ma, G.H. Galactosylated nanocrystallites of insoluble anticancer drug for liver-targeting therapy: An in vitro evaluation. Nanomedicine 2010, 5, 589–596. [Google Scholar] [CrossRef] [PubMed]









| Polymer | DP of lactic acid 1 | Mn (g·mol−1) 2 | Mn (g·mol−1) 3 | PDI 3 | |
|---|---|---|---|---|---|
| Theoretical DP | Actual DP 2 | ||||
| PDLA–PEG–PDLA | 80 | 76 | 9,500 | 17,460 | 1.14 |
| PLLA–PEG–PLLA | 80 | 76 | 9,500 | 17,550 | 1.13 |
| Micelle | CMC (mg·mL−1) | Dh (nm) | DLC (wt %) | DLE (wt %) | |
|---|---|---|---|---|---|
| Blank | Load | ||||
| PDM | 0.072 | 231 ± 4 | 286 ± 6 | 10.16 | 75.41 |
| PLM | 0.061 | 216 ± 5 | 274 ± 5 | 11.93 | 90.29 |
| SCM | 0.035 | 164 ± 4 | 270 ± 5 | 12.96 | 99.22 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Piao, M.; Li, D. Stereocomplex-Reinforced PEGylated Polylactide Micelle for Optimized Drug Delivery. Polymers 2016, 8, 165. https://doi.org/10.3390/polym8040165
Feng C, Piao M, Li D. Stereocomplex-Reinforced PEGylated Polylactide Micelle for Optimized Drug Delivery. Polymers. 2016; 8(4):165. https://doi.org/10.3390/polym8040165
Chicago/Turabian StyleFeng, Chunsheng, Meihua Piao, and Di Li. 2016. "Stereocomplex-Reinforced PEGylated Polylactide Micelle for Optimized Drug Delivery" Polymers 8, no. 4: 165. https://doi.org/10.3390/polym8040165
APA StyleFeng, C., Piao, M., & Li, D. (2016). Stereocomplex-Reinforced PEGylated Polylactide Micelle for Optimized Drug Delivery. Polymers, 8(4), 165. https://doi.org/10.3390/polym8040165