Preparation of Well-Defined Propargyl-Terminated Tetra-Arm Poly(N-isopropylacrylamide)s and Their Click Hydrogels Crosslinked with β-cyclodextrin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
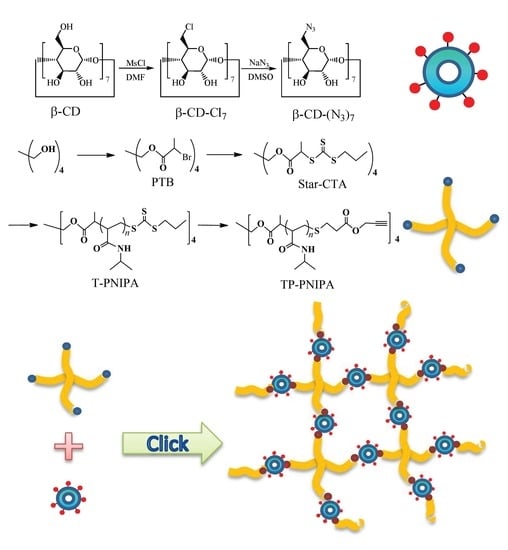
2.2. Preparation of β-CD-(N3)7
2.3. Synthesis of Star-CTA
2.4. RAFT Polymerization of NIPA
2.5. Preparation of Tetra-propargyl Terminal PNIPAs (TP-PNIPAs)
2.6. Estimation of PNIPA Molecular Weights
2.7. Temperature Sensitivities of PNIPAs in Aqueous Solution
2.8. Fabrication of PNIPA Hydrogels
2.9. Swelling Properties of Hydrogels
2.10. Characterization
3. Results and Discussion
3.1. Preparation of Telechelic Tetra-Arm PNIPAs through RAFT Polymerization
3.2. Modification of RAFT PNIPAs
3.3. Temperature Sensitivities of Tetra-Arm PNIPAs in Aqueous Solution
3.4. Fabrication and Temperature Sensitivities of Click PNIPA Hydrogels
3.5. SEM Photographs of Freeze-Dried Hydrogel Networks
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. RAFT polymerization and some of its applications. Chem. Asian J. 2013, 8, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Harvison, M.A.; Lowe, A.B. Combining RAFT radical polymerization and click/highly efficient coupling chemistries: A powerful strategy for the preparation of novel materials. Macromol. Rapid Commun. 2011, 32, 779–800. [Google Scholar] [CrossRef] [PubMed]
- Roth, P.J.; Boyer, C.; Lowe, A.B.; Davis, T.P. RAFT polymerization and thiol chemistry: A complementary pairing for implementing modern macromolecular design. Macromol. Rapid Commun. 2011, 32, 1123–1143. [Google Scholar] [CrossRef] [PubMed]
- Moad, G.; Rizzardo, E.; Thang, S.H. End-functional polymers, thiocarbonylthio group removal/transformation and reversible addition-fragmentation-chain transfer (RAFT) polymerization. Polym. Int. 2011, 60, 9–25. [Google Scholar] [CrossRef]
- Bassik, N.; Abebe, B.T.; Laflin, K.E.; Gracias, D.H. Photolithographically patterned smart hydrogel based bilayer actuators. Polymer 2010, 51, 6093–6098. [Google Scholar] [CrossRef]
- Gu, Y.D.; Dhanarajan, A.P.; Hruby, S.L.; Baldi, A.; Ziaie, B.; Siegel, R.A. An interpenetrating glass-thermosensitive hydrogel construct gated flow control and thermofluidic oscillations. Sens. Actuators B Chem. 2009, 138, 631–636. [Google Scholar] [CrossRef]
- Deshmukh, M.V.; Vaidya, A.A.; Kulkarni, M.G.; Rajamohanan, P.R.; Ganapathy, S. LCST in poly(N-isopropylacrylamide) copolymers: High resolution proton NMR investigations. Polymer 2000, 41, 7951–7960. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H.; Zhang, L.; Yang, G.; Liu, X.; Kim, I. A facile approach for the synthesis of cyclic poly(N-isopropylacrylamide) based on an anthracene–thiol click reaction. Polym. Chem. 2013, 4, 2428. [Google Scholar] [CrossRef]
- Qiu, X.-P.; Korchagina, E.V.; Rolland, J.; Winnik, F.M. Synthesis of a poly(N-isopropylacrylamide) charm bracelet decorated with a photomobile α-cyclodextrin charm. Polym. Chem. 2014, 5, 3656–3665. [Google Scholar] [CrossRef]
- Tao, L.; Kaddis, C.S.; Loo, R.R.O.; Grover, G.N.; Loo, J.A.; Maynard, H.D. Synthesis of maleimide-end-functionalized star polymers and multimeric protein–polymer conjugates. Macromolecules 2009, 42, 8028–8033. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhu, S.Z.; Zhao, H.Y. Brush macromolecules with thermo-sensitive coil backbones and pendant polypeptide side chains: Synthesis, self-assembly and functionalization. Polym. Chem. 2015, 6, 1316–1324. [Google Scholar] [CrossRef]
- Tao, W.; Yan, L.F. Thermogelling of highly branched poly(N-isopropylacrylamide). J. Appl. Polym. Sci. 2010, 118, 3391–3399. [Google Scholar] [CrossRef]
- Lv, W.; Liu, S.; Feng, W.; Qi, J.; Zhang, G.; Zhang, F.; Fan, X. Temperature- and redox-directed multiple self assembly of poly(N-isopropylacrylamide) grafted dextran nanogels. Macromol. Rapid Commun. 2011, 32, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Q.; Wang, L.Q.; Exarhos, G.J.; Li, A.D.Q. Thermosensitive gold nanoparticles. J. Am. Chem. Soc. 2004, 126, 2656–2657. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Qin, G.K.; Olsen, B.D. Highly active biocatalytic coatings from protein-polymer diblock copolymers. ACS Appl. Mater. Interfaces 2015, 7, 14660–14669. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.Y.; Tang, X.Z.; Pan, C.Y. Tadpole-shaped amphiphilic copolymers prepared via RAFT polymerization and click reaction. J. Polym. Sci. Part A 2008, 46, 2390–2401. [Google Scholar] [CrossRef]
- Qiu, X.-P.; Winnik, F.M. Facile and efficient one-pot transformation of RAFT polymer end groups via a mild aminolysis/michael addition sequence. Macromol. Rapid Commun. 2006, 27, 1648–1653. [Google Scholar] [CrossRef]
- Yu, B.; Chan, J.W.; Hoyle, C.E.; Lowe, A.B. Sequential thiol-ene/thiol-ene and thiol-ene/thiol-yne reactions as a route to well-defined mono and bis end-functionalized poly(N-isopropylacrylamide). J. Polym. Sci. Part A 2009, 47, 3544–3557. [Google Scholar] [CrossRef]
- Ooi, H.W.; Jack, K.S.; Peng, H.; Whittaker, A.K. “Click” PNIPAAm hydrogels—A comprehensive study of structure and properties. Polym. Chem. 2013, 4, 4788. [Google Scholar] [CrossRef]
- Ooi, H.W.; Jack, K.S.; Whittaker, A.K.; Peng, H. Photo-initiated thiol-ene “click” hydrogels from RAFT-synthesized poly(N-isopropylacrylamide). J. Polym. Sci. Part A 2013, 51, 4626–4636. [Google Scholar] [CrossRef]
- Pasale, S.K.; Cerroni, B.; Ghugare, S.V.; Paradossi, G. Multiresponsive hyaluronan-P(NIPAAm) “click”-linked hydrogels. Macromol. Biosci. 2014, 14, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Liu, Y.; Lv, Y.; Shao, Z. Poly(N-isopropylacrylamide) hydrogels crosslinked by small-molecular crosslinkers through click chemistry. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 104–110. [Google Scholar] [CrossRef]
- Wang, J.Q.; Kang, Z.Y.; Qi, B.; Zhou, Q.S.; Xiao, S.Y.; Shao, Z.Q. Poly(N-isopropylacrylamide) hydrogels fabricated via click chemistry: Well-defined α,ω-bis propargyl linear poly(N-isopropylacrylamide)s as crosslinkers. RSC Adv. 2014, 4, 51510–51518. [Google Scholar] [CrossRef]
- Le, H.T.; Jeon, H.M.; Lim, C.W.; Kim, T.W. 6-Triazolyl-6-deoxy-β-cyclodextrin derivatives: Synthesis, cellular toxicity, and phase-solubility study. Carbohydr. Res. 2014, 391, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.M.; Ravoo, B.J. Highly fluorinated cyclodextrins and their host-guest interactions. Chem. Commun. 2010, 46, 4369–4371. [Google Scholar] [CrossRef] [PubMed]
- Zayas, H.A.; Truong, N.P.; Valade, D.; Jia, Z.; Monteiro, M.J. Narrow molecular weight and particle size distributions of polystyrene 4-arm stars synthesized by RAFT-mediated miniemulsions. Polym. Chem. 2013, 4, 592. [Google Scholar] [CrossRef]
- Xu, J.; Ye, J.; Liu, S.Y. Synthesis of well-defined cyclic poly(N-isopropylacrylamide) via click chemistry and its unique thermal phase transition behavior. Macromolecules 2007, 40, 9103–9110. [Google Scholar] [CrossRef]
- Qiu, X.P.; Tanaka, F.; Winnik, F.M. Temperature-induced phase transition of well-defined cyclic poly(N-isopropylacrylamide)s in aqueous solution. Macromolecules 2007, 40, 7069–7071. [Google Scholar] [CrossRef]
- Jain, K.; Vedarajan, R.; Watanabe, M.; Ishikiriyama, M.; Matsumi, N. Tunable LCST behavior of poly(N-isopropylacrylamide/ionic liquid) copolymers. Polym. Chem. 2015, 6, 6819–6825. [Google Scholar] [CrossRef]
- Fujishige, S.; Kubota, K.; Ando, I. Phase-transition of aqueous-solutions of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide). J. Phys. Chem. Us 1989, 93, 3311–3313. [Google Scholar] [CrossRef]
- Chen, Y.G.; Xiao, N.; Fukuoka, M.; Yoshida, K.; Duan, Q.; Satoh, T.; Kakuchi, T. Synthesis and thermoresponsive properties of four-arm star-shaped poly(N-isopropylacrylamide)s bearing covalent and non-covalent cores. Polym. Chem. 2015, 6, 3608–3616. [Google Scholar] [CrossRef]
- Xia, Y.; Burke, N.A.D.; Stover, H.D.H. End group effect on the thermal response of narrow-disperse poly(N-isopropylacrylamide) prepared by atom transfer radical polymerization. Macromolecules 2006, 39, 2275–2283. [Google Scholar] [CrossRef]
- Xia, Y.; Yin, X.C.; Burke, N.A.D.; Stover, H.D.H. Thermal response of narrow-disperse poly(N-isopropylacrylamide) prepared by atom transfer radical polymerization. Macromolecules 2005, 38, 5937–5943. [Google Scholar] [CrossRef]
- Lessard, D.G.; Ousalem, M.; Zhu, X.X. Effect of the molecular weight on the lower critical solution temperature of poly(N,N-diethylacrylamide) in aqueous solutions. Can. J. Chem. 2001, 79, 1870–1874. [Google Scholar] [CrossRef]
- Zheng, X.; Tong, Z.; Xie, X.L.; Zeng, F. Phase separation in poly(N-isopropyl acrylamide) water solutions I. Cloud point curves and microgelation. Polym. J. 1998, 30, 284–288. [Google Scholar] [CrossRef]
- Plummer, R.; Hill, D.J.T.; Whittaker, A.K. Solution properties of star and linear poly(N-isopropylacrylamide). Macromolecules 2006, 39, 8379–8388. [Google Scholar] [CrossRef]
- Kujawa, P.; Segui, F.; Shaban, S.; Diab, C.; Okada, Y.; Tanaka, F.; Winnik, F.M. Impact of end-group association and main-chain hydration on the thermosensitive properties of hydrophobically modified telechelic poly(N-isopropylacrylamides) in water. Macromolecules 2006, 39, 341–348. [Google Scholar] [CrossRef]
- Xu, X.D.; Chen, C.S.; Wang, Z.C.; Wang, G.R.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. “Click” chemistry for in situ formation of thermoresponsive P(NIPAAm-co-HEMA)-based hydrogels. J. Polym. Sci. Part A 2008, 46, 5263–5277. [Google Scholar] [CrossRef]
- Xu, X.D.; Chen, C.S.; Lu, B.; Wang, Z.C.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Modular synthesis of thermosensitive P(NIPAAm-co-HEMA)/β-CD based hydrogels via click chemistry. Macromol. Rapid Commun. 2009, 30, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Shen, Y.; Li, X.; Cai, S.; Liu, M. Synthesis and characterization of thermo- and pH-sensitive poly(vinyl alcohol)/poly(N,N-diethylacrylamide-co-itaconic acid) semi-IPN hydrogels. Biomed. Mater. 2012. [Google Scholar] [CrossRef] [PubMed]







| Samples | [NIPA]/[Star- CTA]/[AIBN] | Polymerization time (min) | Conversion (%) | Mn (kg/mol) | PDI (GPC) | ||
|---|---|---|---|---|---|---|---|
| Theory a | 1H NMR b | UV–Vis c | |||||
| T-PNIPA7K | 80/1/0.2 | 120 | 68 | 7.1 | 6.7 | 6.8 | 1.14 |
| T-PNIPA16K | 167/1/0.2 | 240 | 84 | 16.8 | 16.3 | 17.3 | 1.12 |
| T-PNIPA24K | 250/1/0.2 | 270 | 82 | 24.1 | 25.4 | 23.5 | 1.18 |
| Samples | Mn a (kg/mol) | PDI | TLCST,min (°C) | TLCST,max (°C) | ΔT (°C) | TCP b (°C) |
|---|---|---|---|---|---|---|
| TP-PNIPA7K | 7.4 | 1.16 | 25.5 | 33.0 | 7.5 | 28.5 |
| TP-PNIPA16K | 16.5 | 1.18 | 27.8 | 32.0 | 4.2 | 29.0 |
| TP-PNIPA24K | 25.0 | 1.20 | 30.0 | 33.5 | 3.5 | 32.0 |
| T-PNIPA16K | 16.3 | 1.12 | 25.2 | 31.0 | 5.8 | 27.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhu, Z.; Jin, X.; Li, Z.; Shao, Y.; Shao, Z. Preparation of Well-Defined Propargyl-Terminated Tetra-Arm Poly(N-isopropylacrylamide)s and Their Click Hydrogels Crosslinked with β-cyclodextrin. Polymers 2016, 8, 93. https://doi.org/10.3390/polym8040093
Wang J, Zhu Z, Jin X, Li Z, Shao Y, Shao Z. Preparation of Well-Defined Propargyl-Terminated Tetra-Arm Poly(N-isopropylacrylamide)s and Their Click Hydrogels Crosslinked with β-cyclodextrin. Polymers. 2016; 8(4):93. https://doi.org/10.3390/polym8040093
Chicago/Turabian StyleWang, Jianquan, Zhe Zhu, Xin Jin, Zhujun Li, Yizhen Shao, and Ziqiang Shao. 2016. "Preparation of Well-Defined Propargyl-Terminated Tetra-Arm Poly(N-isopropylacrylamide)s and Their Click Hydrogels Crosslinked with β-cyclodextrin" Polymers 8, no. 4: 93. https://doi.org/10.3390/polym8040093
APA StyleWang, J., Zhu, Z., Jin, X., Li, Z., Shao, Y., & Shao, Z. (2016). Preparation of Well-Defined Propargyl-Terminated Tetra-Arm Poly(N-isopropylacrylamide)s and Their Click Hydrogels Crosslinked with β-cyclodextrin. Polymers, 8(4), 93. https://doi.org/10.3390/polym8040093