Synthesis and Mechanism of Metal-Mediated Polymerization of Phenolic Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
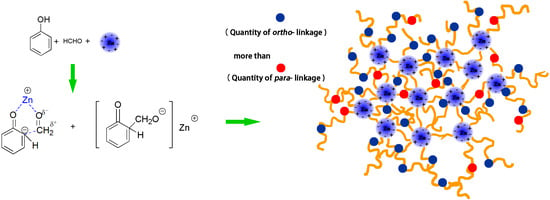
2.2. Preparation of PF Resins
2.3. Preparation of Plywood
2.4. Characterization of PF Resins
2.5. Characterization of the Plywood
2.6. FT-IR Analysis of PF Resins
2.7. Contact Angle Measurement
2.8. Quantitative Liquid 13C NMR Measurement
2.9. Thermogravimetric Analysis (TG) of Resins
3. Results and Discussion
3.1. Performance of the Catalyst-Accelerated PF Resin
3.2. Contact Angle of the PF Resins
3.3. FT-IR Spectroscopy
3.4. Chemical Structure Analysis
3.5. Plywood Performance
3.6. Thermal Behavior of the Cured PF Resins
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hirano, K.; Asami, M. Phenolic resins-100 years of progress and their future. React. Funct. Polym. 2013, 73, 256–269. [Google Scholar] [CrossRef]
- Nair, C.P.R. Advances in addition-cure phenolic resins. Prog. Polym. Sci. 2004, 29, 401–498. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, Q.; Lian, K. Cure kinetics of aqueous phenol-formaldehyde resins used for oriented strandboard manufacturing: Analytical technique. J. Appl. Polym. Sci. 2006, 100, 1642–1650. [Google Scholar] [CrossRef]
- Zhao, C.; Pizzi, A.; Garnier, S. Fast advancement and hardening acceleration of low-condensation alkaline PF resins by esters and copolymerized urea. J. Appl. Polym. Sci. 1999, 74, 359–378. [Google Scholar] [CrossRef]
- Lei, H.; Pizzi, A.; Despres, A.; Pasch, H.; Du, G. Ester acceleration mechanisms in phenol–Formaldehyde resin adhesives. J. Appl. Polym. Sci. 2006, 100, 3075–3093. [Google Scholar] [CrossRef]
- Pizzi, A.; Stephanou, A. Completion of alkaline cure acceleration of phenol–formaldehyde resins: Acceleration by organic anhydrides. J. Appl. Polym. Sci. 1994, 51, 1351–1352. [Google Scholar] [CrossRef]
- Gabilondo, N.; Lopez, M.; Ramos, J.A.; Echeverria, J.M.; Mondragon, I. Curing kinetics of amine and sodium hydroxide catalyzed phenol-formaldehyde resins. J. Therm. Anal. Calorim. 2007, 90, 229–236. [Google Scholar] [CrossRef]
- Park, B.-D.; Riedl, B.; Hsu, E.W.; Shields, J. Differential scanning calorimetry of phenol–formaldehyde resins cure-accelerated by carbonates. Polymer 1999, 40, 1689–1699. [Google Scholar] [CrossRef]
- Grenier-Loustalot, M.-F.; Larroque, S.; Grande, D.; Grenier, P.; Bedel, D. Phenolic resins: 2. Influence of catalyst type on reaction mechanisms and kinetics. Polymer 1996, 37, 1363–1369. [Google Scholar] [CrossRef]
- Pizzi, A.; Garcia, R.; Wang, S. On the networking mechanisms of additives-accelerated phenol–formaldehyde polycondensates. J. Appl. Polym. Sci. 1997, 66, 255–266. [Google Scholar] [CrossRef]
- Paju, J.; Pehk, T.; Christjanson, P. Structure of phenol-formaldehyde polycondensates. Proc. Estonian Acad. Sci. 2009, 58, 45–52. [Google Scholar] [CrossRef]
- He, G.B.; Yan, N. 13C NMR study on structure, composition and curing behavior of phenol-urea-formaldehyde resole resins. Polymer 2004, 45, 6813–6822. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Kim, H.-J.; Rafailovich, M.; Sokolov, J. Curing monitoring of phenolic resol resins via atomic force microscope and contact angle. Int. J. Adhes. Adhes. 2002, 22, 375–384. [Google Scholar] [CrossRef]
- Shi, S.Q.; Gardner, D.J. Dynamic adhesive wettability of wood. Wood Fiber Sci. 2001, 33, 58–68. [Google Scholar]
- Chu, P.P.; Reddy, M.J.; Tsai, J. Structural and transport characteristics of polyethylene oxide/phenolic resin blend solid polymer electrolytes. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3866–3875. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Y.F.; Xu, Y.Z.; Wang, C.P.; Chu, F.X. Lignocellulosic ethanol residue-based lignin-phenol-formaldehyde resin adhesive. Int. J. Adhes. Adhes. 2013, 40, 11–18. [Google Scholar] [CrossRef]
- Lin, C.T.; Lee, H.T.; Chen, J.K. Preparation and properties of bisphenol-F based boron-phenolic resin/modified silicon nitride composites and their usage as binders for grinding wheels. Appl. Surf. Sci. 2015, 330, 1–9. [Google Scholar] [CrossRef]
- Park, B.-D.; Riedl, B. 13C-NMR study on cure-accelerated phenol-formaldehyde resins with carbonates. J. Appl. Polym. Sci. 2000, 77, 1284–1293. [Google Scholar] [CrossRef]
- Vázquez, G.; López-Suevos, F.; Villar-Garea, A.; González-Alvarez, J.; Antorrena, G. 13C-NMR analysis of phenol-urea-formaldehyde prepolymers and phenol-urea-formaldehyde-tannin adhesives. J. Adhes. Sci. Technol. 2004, 18, 1529–1543. [Google Scholar] [CrossRef]
- Fan, D.; Chang, J.; Li, J.; Mao, A.; Zhang, L. 13C-NMR study on the structure of phenol-urea-formaldehyde resins prepared by methylolureas and phenol. J. Appl. Polym. Sci. 2009, 112, 2195–2202. [Google Scholar] [CrossRef]
- Alma, M.H.; Kelley, S.S. Thermal stability of novolak-type thermosettings made by the condensation of bark and phenol. Polym. Degrad. Stab. 2000, 68, 413–418. [Google Scholar] [CrossRef]
- Yi, Z.; Li, C.; Jiang, J.X.; Zhang, J.Z.; Zhang, W.; Li, J.Z. Pyrolysis kinetics of tannin-phenol-formaldehyde resin by non-isothermal thermogravimetric analysis. J. Therm. Anal. Calorim. 2015, 121, 867–876. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Su, W.C.; Lin, Y.C.; Wang, C.F.; Chen, J.K.; Jeong, K.U.; Kuo, S.W. Azopyridine-functionalized benzoxazine with Zn(ClO4)(2) form high-performance polybenzoxazine stabilized through metal-ligand coordination. RSC Adv. 2014, 4, 50373–50385. [Google Scholar] [CrossRef]
- Pizzi, A. Phenolic resins by reactions of coordinated metal ligands. J. Polym. Sci. Polym. Lett. Ed. 1979, 17, 489–492. [Google Scholar] [CrossRef]
- Pizzi, A. Phenolic and tannin-based adhesive resins by reactions of coordinated metal ligands. I. Phenolic chelates. J. Appl. Polym. Sci. 1979, 24, 1247–1255. [Google Scholar] [CrossRef]








| Catalyst type | Performance | ||
|---|---|---|---|
| Solid content (%) | Viscosity (mPa·s) | Gel time (min) | |
| Control | 43 | 25.70 | 20.46 |
| Ba(OH)2 | 46 | 73.70 | 15.57 |
| Na2CO3 | 44 | 153.00 | 11.83 |
| LiOH | 46 | 58.30 | 15.88 |
| (CH3COO)2Zn | 44 | 81.00 | 13.98 |
| Wavenumbers (cm−1) | Assignment |
|---|---|
| 3,367 | –OH stretching vibration |
| 2,900 | C–H stretching vibration of methylene |
| 1,600, 1440 | The elongation of aromatic –C=C– |
| 1,270 | C–O stretching vibration of phenolic C–OH and phenolic C–O |
| 1,020 | C–O stretching vibration of aliphatic C–OH, aliphatic C–O, and methylol C–OH |
| 970 | C–H stretching vibration of vinyl |
| Wavenumbers (cm−1) | Absorption | ||||
|---|---|---|---|---|---|
| Control | Ba(OH)2 | Na2CO3 | LiOH | (CH3COO)2Zn | |
| 1,020 | 43.46 | 32.63 | 33.79 | 45.43 | 29.85 |
| 1,600 | 29.75 | 28.14 | 29.94 | 38.54 | 24.81 |
| Ratio (1,020/1,600) | 1.46 | 1.16 | 1.13 | 1.17 | 1.20 |
| PF resin | ortho/para (Substituted position) | ortho/para (Methylol) | ortho-para/para–para (Methylene bridges) |
|---|---|---|---|
 |  |  |  |
| Control |  |  |  |
| Ba(OH)2 | |||
| Na2CO3 | |||
| LiOH | |||
| (CH3COO)2Zn | |||
| Zn(NO3)2 |
| Catalyst type | Tmax of Thermal event (°C) | Weight residue (%) at 700 °C | ||||
|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | Fifth | ||
| Control | 155 | 260 | 394 | 507 | – | 65.5 |
| Ba(OH)2 | 156 | 262 | 390 | 503 | – | 68.0 |
| Na2CO3 | 153 | 300 | 386 | 512 | – | 65.5 |
| LiOH | 158 | 283 | 381 | 497 | – | 68.0 |
| (CH3COO)2Zn | 155 | 273 | 381 | 493 | 518 | 68.0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Z.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Synthesis and Mechanism of Metal-Mediated Polymerization of Phenolic Resins. Polymers 2016, 8, 159. https://doi.org/10.3390/polym8050159
Yi Z, Zhang J, Zhang S, Gao Q, Li J, Zhang W. Synthesis and Mechanism of Metal-Mediated Polymerization of Phenolic Resins. Polymers. 2016; 8(5):159. https://doi.org/10.3390/polym8050159
Chicago/Turabian StyleYi, Zhao, Jizhi Zhang, Shifeng Zhang, Qiang Gao, Jianzhang Li, and Wei Zhang. 2016. "Synthesis and Mechanism of Metal-Mediated Polymerization of Phenolic Resins" Polymers 8, no. 5: 159. https://doi.org/10.3390/polym8050159
APA StyleYi, Z., Zhang, J., Zhang, S., Gao, Q., Li, J., & Zhang, W. (2016). Synthesis and Mechanism of Metal-Mediated Polymerization of Phenolic Resins. Polymers, 8(5), 159. https://doi.org/10.3390/polym8050159