Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Sodium Alginate–Chitosan Hydrosols
2.3. Hydrogel Film Formation
2.4. Thermal Gravimetric Analysis (TGA)
2.5. Dynamical Mechanical Thermo Analysis (DMTA)
2.6. Nuclear Magnetic Resonance (NMR)
2.7. Fourier Transform Infrared Spectroscopy (FT-IR)
2.8. Matrix-Assisted Laser Desorption/Ionization Technique with Time of Flight Analyzer (Maldi-Tof)
2.9. Scanning Electron Microscopy
2.10. Statistical Analysis
3. Results and Discussion
3.1. pH Measurement
3.2. Thermogravimetric Analysis (TGA)
3.3. Dynamic Mechanical Thermal Analysis (DMTA)
3.4. Nuclear Magnetic Resonance (NMR)
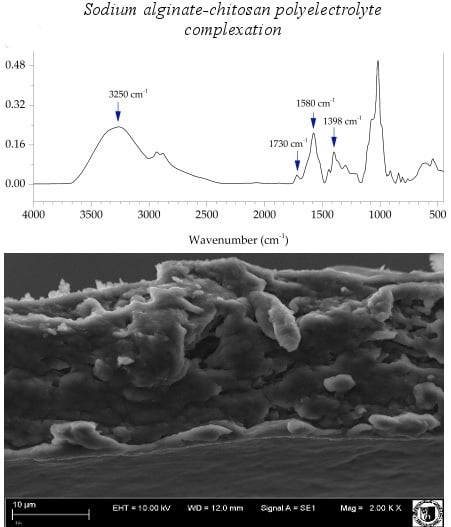
3.5. FT-IR Analysis
3.6. Matrix-Assisted Laser Desorption/Ionization Technique with Time of Flight Analyzer (MALDI-TOF)
3.7. Scanning Electron Microstructure (SEM)
4. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Hunkeler, D. Alginate-oligochitosan microcapsules: A mechanistic study relating membrane and capsule properties to reaction conditions. Chem. Mater. 1999, 11, 2486–2492. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Brylak, W. Hydrożelowe mikrokapsułki z udziałem naturalnych i chemicznie modyfikowanych oligochitozanów—Właściwości mechaniczne i porowatość. Polimery 2006, 51, 547–554. [Google Scholar]
- Sæther, H.V.; Holme, H.K.; Maurstad, G.; Smidsrød, O.; Stokke, B.T. Polyelectrolyte complex formation using alginate and chitosan. Carbohydr. Polym. 2008, 74, 813–821. [Google Scholar] [CrossRef]
- Thünemann, A.F.; Müller, M.; Dautzenberg, H.; Joanny, J.F.; Löwen, H. Polyelectrolyte complexes. Adv. Polym. Sci. 2004, 166, 113–171. [Google Scholar]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Haug, A.; Smidsrød, O. Strontium-calcium selectivity of alginates. Nature 1967, 215, 757. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Smidsrød, O. Selectivity of some anionic polymers for divalent metal ions. Acta Chem. Scand. 1970, 24, 843–854. [Google Scholar] [CrossRef]
- Smidsrød, O. Molecular basis of some physical properties of alginates in the gel state. Faraday Discuss. Chem. Soc. 1974, 57, 263–274. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Chitosan-sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 2005, 41, 1859–1866. [Google Scholar] [CrossRef]
- Daly, M.M.; Knorr, D. Chitosan-alginate complex coacervate capsules—Effects of calcium-chloride, plasticizers, and polyelectrolytes on mechanical stability. Biotechnol. Prog. 1988, 4, 6–81. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interaction between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, I.R.; Miraftab, M.; Collyer, G. Absorbent alginate fibres modified with hydrolysed chitosan for wound care dressings—II. Pilot scale development. Carbohydr. Polym. 2014, 102, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Reversed chitosan-alginate polyelectrolyte complex for stability improvement of α-amylase: Optimization and physicochemical characterization. Eur. J. Pharm. Biopharm. 2007, 65, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 18, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Carvalho, S.; Quintas, M.A.C.; Teixeira, A.; Vicente, A.A. Physical and thermal properties of a chitosan/alginate nanolayered PET film. Carbohydr. Polym. 2010, 82, 153–159. [Google Scholar] [CrossRef]
- Guizhen, K.; Weilin, X.; Weidong, Y. Preparation and properties of drug-loaded chitosan-sodium alginate complex membrane. Int. J. Polym. Mater. Polym. Biomater. 2010, 59, 184–191. [Google Scholar]
- Costa, R.R.; Costa, A.M.S.; Caridade, S.G.; Mano, J.F. Compact saloplastic membranes of natural polysaccharides for soft tissue engineering. Chem. Mater. 2015, 27, 7490–7502. [Google Scholar] [CrossRef]
- Guzmán, E.; Chuliá-Jordán, R. Influence of the percentage of acetylation on the assembly of LbL multilayers of poly(acrylic acid) and chitosan. Phys. Chem. Chem. Phys. 2011, 13, 18200–18207. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Cavallo, J.A.; Chuliá-Jordán, R.; Gómez, C.; Strumia, M.C.; Ortega, F.; Rubio, R.G. pH-Induced changes in the fabrication of multilayers of poly(acrylic acid) and chitosan: Fabrication, properties, and tests as a drug storage and delivery system. Langmuir 2011, 27, 6836–6845. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yao, F. Environment-stimuli response of chitosan-based hydrogels. In Chitosan-Based Hydrogels: Functions and Applications; Yao, K., Li, J., Yao, F., Yin, Y., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 239–241. [Google Scholar]
- Neto, C.G.T.; Giacometti, J.A.; Job, A.E.; Ferreira, F.C.; Fonseca, J.L.C.; Pereira, M.R. Thermal analysis of chitosan based networks. Carbohydr. Polym. 2005, 62, 97–103. [Google Scholar] [CrossRef]
- Fedi, S.; Barbucci, R. Physical and chemical hyaluronic acid hydrogels and their biomedical applications. In Polysaccharide Hydrogels: Characterization and Biomedical Applications; Matricardi, P., Alhaique, F., Coviello, T., Eds.; Pan Stanford Publishing: Singapore, 2015; Volume 12, pp. 417–448. [Google Scholar]
- Salomonsen, T.; Jensen, H.M.; Larsen, F.H.; Steuernagel, S.; Engelsen, S.B. Direct quantification of M/G ratio from 13C CP-MAS NMR spectra of alginate powders by multivariate curve resolution. Carbohydr. Res. 2009, 344, 2014–2022. [Google Scholar] [CrossRef] [PubMed]
- Jančiauskaitė, U.; Višnevskij, C.; Radzevičius, K.; Makuška, R. Polyampholytes from natural building blocks: Synthesis and properties of chitosan-o-alginate copolymers. Chemija 2009, 20, 128–135. [Google Scholar]
- Han, J.H. Edible films and coatings: A review. In Innovations in Food Packaging; Elsevier: Cambridge, MA, USA, 2014; pp. 222–223. [Google Scholar]
- Leceta, I.; Guerrero, P.; de la Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.K.S.; Pinto, L.F.; Gomes, C.A.R.; Gomes, P. Novel highly-soluble peptide-chitosan polymers: Chemical synthesis and spectral characterization. Carbohydr. Polym. 2006, 64, 299–305. [Google Scholar] [CrossRef]
- Meng, X.; Tian, F.; Yang, J.; He, C.N.; Xing, N.; Li, F. Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. Mater. Sci. Mater. Med. 2010, 21, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Le-Tien, C.; Millette, M.; Mateescu, M.A.; Lacroix, M. Modified alginate and chitosan for lactic acid bacteria. Immobil. Biotechnol. Appl. Biochem. 2004, 39, 347–354. [Google Scholar]
- Drogoz, A.; David, L.; Rochas, C.; Domard, A.; Delair, T. Polyelectrolyte complexes from polysaccharides: Formation and stoichiometry monitoring. Langmuir 2007, 23, 10950–10958. [Google Scholar] [CrossRef] [PubMed]
- Schatz, C.; Domard, A.; Viton, C.; Pichot, C.; Delair, T. Versatile and efficient formation of colloids of biopolymer-based polyelectrolyte complexes. Biomacromolecules 2004, 5, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Arzate-Vázquez, I.; Chanona-Pérez, J.J.; Calderón-Domínguez, G.; Eduardo Terres-Rojas, E.; Garibay-Febles, V.; Martínez-Riva, A.; Gutiérrez-López, G.F. Microstructural characterization of chitosan and alginate films by microscopy techniques and texture image analysis. Carbohydr. Polym. 2012, 87, 289–299. [Google Scholar] [CrossRef]










| Variants | Volume Proportions of Hydrosols | Polymers Ratio |
|---|---|---|
| ALG/CH | R | |
| ACH-1 | 3/15 | 0.20 |
| ACH-2 | 4/15 | 0.27 |
| ACH-3 | 3/10 | 0.30 |
| ACH-4 | 5/15 | 0.33 |
| ACH-5 | 4/10 | 0.40 |
| ACH-6 | 5/10 | 0.50 |
| ACH-7 | 3/5 | 0.60 |
| ACH-8 | 4/5 | 0.80 |
| ACH-9 | 5/5 | 1.00 |
| ALG | 1/0 | - |
| CH | 0/1 | - |
| Sample | Inflection Point 1 (°C) | Inflection Point 2 (°C) | Inflection Point 3 (°C) | Inflection Point 4 (°C) |
|---|---|---|---|---|
| ALG | 64.8 ± 0.4 e | 162.5 ± 0.8 c | N | N |
| CH | 71.7 ± 0.3 g | 181.3 ± 0.7 a | N | 279.2 ± 0.5 d |
| ACH-1 | 60.1 ± 0.2 e | 180.9 ± 0.4 a | N | 275.6 ± 0.6 c |
| ACH-2 | 58.1 ± 0.3 c | 182.5 ± 0.5 b | N | 270.5 ± 0.2 a |
| ACH-3 | 56.7 ± 0.3 a | 180.9 ± 0.7 a | 211.8 ± 0.6 c | N |
| ACH-4 | 58.4 ± 0.8 c | 191.2 ± 0.8 f | N | 273.5 ± 0.6 b |
| ACH-5 | 56.9 ± 0.5 a | 183.7 ± 0.7 e | 208.7 ± 0.2 b | N |
| ACH-6 | 54.0 ± 0.5 b | 182.6 ± 0.4 b | 222.2 ± 0.3 a | N |
| ACH-7 | 55.0 ± 0.4 d | 181.2 ± 0.6 a | 222.4 ± 0.5 a | N |
| ACH-8 | 56.3 ± 0.4 a | 178.5 ± 0.3 d | 222.5 ± 0.4 a | N |
| ACH-9 | 54.2 ± 0.8 b | 182.6 ± 0.3 b | 222.1 ± 0.5 a | N |
| Sample | Mass Loss 1 (%) | Mass Loss 2 (%) | Mass Loss 3 (%) | Mass Loss 4 (%) | Final Residue (%) |
|---|---|---|---|---|---|
| ALG | 13.6 ± 0.7 g | 46.91 ± 0.5 c | N | N | 40.4 ± 0.2 d |
| CH | 13.6 ± 0.5 g | 23.19 ± 0.5 d | N | 24.2 ± 0.7 c | 40.3 ± 0.4 d |
| ACH-1 | 10.1 ± 0.3 e | 45.12 ± 0.2 a | N | 14.3 ± 0.5 a | 30.2 ± 0.2 a,b |
| ACH-2 | 9.3 ± 0.3 b–d | 46.85 ± 0.3 c | N | 14.0 ± 0.7 a | 30.5 ± 0.2 a,b |
| ACH-3 | 9.6 ± 0.4 c–e | 44.92 ± 0.2 a | 15.3 ± 0.5 b | N | 30.7 ± 0.1 a |
| ACH-4 | 8.6 ± 0.5 a,b | 44.5 ± 0.5 a | N | 15.2 ± 0.9 b | 30.9 ± 0.7 a |
| ACH-5 | 11.2 ± 0.4 f | 31.8 ± 0.5 b | 17.5 ± 0.5 d | N | 29.4 ± 0.4 c |
| ACH-6 | 8.9 ± 0.3 a–c | 38.7 ± 0.3 f | 22.1 ± 0.4 a | N | 29.9 ± 0.4 b,c |
| ACH-7 | 11.1 ± 0.4 f | 31.3 ± 0.6 b | 28.9 ± 0.4 e | N | 27.9 ± 0.6 e |
| ACH-8 | 8.4 ± 0.3 a | 40.0 ± 0.4 g | 20.10 ± 0.3 c | N | 30.9 ± 0.4 a |
| ACH-9 | 9.7 ± 0.3 d,e | 37.8 ± 0.3 e | 22.28 ± 0.3 a | N | 29.9 ± 0.6 b,c |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio. Polymers 2016, 8, 167. https://doi.org/10.3390/polym8050167
Kulig D, Zimoch-Korzycka A, Jarmoluk A, Marycz K. Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio. Polymers. 2016; 8(5):167. https://doi.org/10.3390/polym8050167
Chicago/Turabian StyleKulig, Dominika, Anna Zimoch-Korzycka, Andrzej Jarmoluk, and Krzysztof Marycz. 2016. "Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio" Polymers 8, no. 5: 167. https://doi.org/10.3390/polym8050167
APA StyleKulig, D., Zimoch-Korzycka, A., Jarmoluk, A., & Marycz, K. (2016). Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio. Polymers, 8(5), 167. https://doi.org/10.3390/polym8050167