Production and Characterization of a Novel, Electrospun, Tri-Layer Polycaprolactone Membrane for the Segregated Co-Culture of Bone and Soft Tissue
Abstract
:1. Introduction
2. Materials and Methods
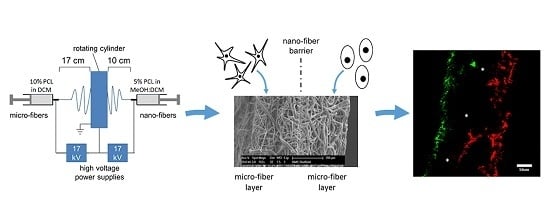
2.1. PCL Scaffold Fabrication
2.2. Scanning Electron Microscopy (SEM) Characterization of Scaffolds
2.3. Cell Culture
2.4. Assessment of Cell Viability of hES-MPs on Electrospun PCL Scaffold
2.5. hES-MPs Cell Line Seeding and Proliferation on Tri-Layer Electrospun PCL Scaffold
2.6. Co-Cultured between hBMSCs and Fibroblasts on Tri-Layer Electrospun PCL Scaffold
3. Results and Discussion
3.1. PCL Tri-Layer Scaffold Fabrication
3.2. Progenitor Cell Seeding and Proliferation on Tri-Layer Electrospun PCL Scaffolds
3.3. Co-Culture between hBMSCs and Fibroblasts on Tri-Layer Electrospun PCL Scaffolds
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roman, S.; Mangera, A.; Osman, N.I.; Bullock, A.J.; Chapple, C.R.; MacNeil, S. Developing a tissue engineered repair material for treatment of stress urinary incontinence and pelvic organ prolapse—Which cell source? Neurourol. Urodyn. 2014, 33, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Hibi, H.; Yamada, Y.; Ueda, M.; Endo, Y. Alveolar cleft osteoplasty using tissue-engineered osteogenic material. Int. J. Oral Maxillofac. Surg. 2006, 35, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Behnia, H.; Khojasteh, A.; Soleimani, M.; Tehranchi, A.; Khoshzaban, A.; Keshel, S.H.; Atashi, R. Secondary repair of alveolar clefts using human mesenchymal stem cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, E1–E6. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Chapple, C.R.; Bullock, A.J.; Layton, C.; MacNeil, S. Tissue-engineered buccal mucosa for substitution urethroplasty. BJU Int. 2004, 93, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Trautvetter, W.; Kaps, C.; Schmelzeisen, R.; Sauerbier, S.; Sittinger, M. Tissue-engineered polymer-based periosteal bone grafts for maxillary sinus augmentation: Five-year clinical results. J. Oral Maxillofac. Surg. 2011, 69, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Delaine-Smith, R.M.; MacNeil, S.; Reilly, G.C. Matrix production and collagen structure are enhance in two types of osteogenic progenitor cells by a simple fluid shear stress stimulus. Eur. Cells Mater. 2012, 24, 162–174. [Google Scholar]
- Sittichokechaiwut, A.; Edwards, J.H.; Scutt, A.M.; Reilly, G.C. Short bouts of mechanical loading are effective as dexamethasone at inducing matrix production by human bone marrow mesenchymal stem cells. Eur. Cells Mater. 2010, 20, 45–57. [Google Scholar]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.W.; Zhu, M.F.; Yang, W.; Yu, H.; Chen, Y.M.; Zhang, Y. Experimental study on relationship between jet instability and formation of beaded fibers during electrospinning. Polym. Eng. Sci. 2005, 45, 704–709. [Google Scholar] [CrossRef]
- De Peppo, G.M.; Svensson, S.; Lenneras, M.; Synnergren, J.; Stenberg, J.; Strehl, R.; Hyllner, J.; Thomsen, P.; Karlsson, C. Human embryonic mesodermal progenitors highly resemble human mesenchymal stem cells and display high potential for tissue engineering applications. Tissue Eng. Part A 2010, 16, 2161–2182. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.T.; Liu, Y.; Advincula, M.; Wang, Y.-H.; Maye, P.; Goldberg, A.J. A nondestructive method for evaluating in vitro osteoblast differentiation on biomaterials using osteoblast-specific fluorescence. Tissue Eng. Part C 2010, 16, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Liu, Y.; Maye, P.; Rowe, D.W. Examination of mineralized nodule formation in living osteoblastic cultures using fluorescent dyes. Biotechnol. Prog. 2006, 22, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Shishatskaya, E.I.; Volova, T.G.; Puzyr, A.P.; Mogilnaya, O.A.; Efremov, S.N. Tissue response to the implantation of biodegradable polyhydroxyalkanoate sutures. J. Mater. Sci. Mater. Med. 2004, 15, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Bye, F.J.; Bissoli, J.; Black, L.; Bullock, A.J.; Puwanun, S.; Moharamzadeh, K.; Reilly, G.C.; Ryan, A.J.; MacNeil, S. Development of bilayer and trilayer nanofibrous/microfibrous scaffolds for regenerative medicine. Biomater. Sci. 2013, 1, 942–951. [Google Scholar] [CrossRef]
- Puwanun, S. Developing a Tissue Engineering Strategy for Cleft Palate Repair. Ph.D. Thesis, the University of Sheffield, Sheffield, UK, 2014. [Google Scholar]
- Selim, M.; Bullock, A.J.; Blackwood, K.A.; Chapple, C.R.; MacNeil, S. Developing biodegradable scaffolds for tissue engineering of the urethra. BJU Int. 2011, 107, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, K.A.; McKean, R.; Canton, I.; Freman, C.O.; Franklin, K.L.; Cole, D.; Brook, I.; Frathing, P.; Rimmer, S.; Haycock, J.W.; et al. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials 2008, 29, 3091–3104. [Google Scholar] [CrossRef] [PubMed]
- Bolgen, N.; Vargel, I.; Korkusuz, P.; Menceloglu, Y.Z.; Piskin, E. In vivo performance of antibiotic embedded electrospun PCL membranes for prevention of abdominal adhesions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81B, 530–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, J.; Sarker, P.; Rimmer, S.; Swanson, L.; MacNeil, S.; Douglas, I. Hyperbranched poly(NIPAM) polymers modified with antibiotics for the reduction of bacterial burden in infected human tissue engineered skin. Biomaterials 2011, 32, 258–267. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, G.; Schiller, P.C.; Ricordi, C.; Roos, B.A.; Howard, G.A. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J. Bone Miner. Res. 1999, 14, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Shishatskaya, E.I.; Nikolaeva, E.D.; Sinskey, A.J. In vivo study of 2D PHA matrices of different chemical compositions: Tissue reactions and biodegradations. Mater. Sci. Technol. 2014, 30, 549–557. [Google Scholar] [CrossRef]
- Delaine-Smith, R.M.; Green, N.H.; Matcher, S.J.; MacNeil, S.; Reilly, G.C. Monitoring fibrous scaffold guidance of three-dimensional collagen organisation using minimally-invasive second harmonic generation. PLoS ONE 2014, 9, e89761. [Google Scholar]
- De Valence, S.; Tille, J.-C.; Mugnai, D.; Mrowczynski, W.; Gurny, R.; Moeller, M.; Walpoth, B.H. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 2012, 33, 38–47. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puwanun, S.; Bye, F.J.; Ireland, M.M.; MacNeil, S.; Reilly, G.C.; Green, N.H. Production and Characterization of a Novel, Electrospun, Tri-Layer Polycaprolactone Membrane for the Segregated Co-Culture of Bone and Soft Tissue. Polymers 2016, 8, 221. https://doi.org/10.3390/polym8060221
Puwanun S, Bye FJ, Ireland MM, MacNeil S, Reilly GC, Green NH. Production and Characterization of a Novel, Electrospun, Tri-Layer Polycaprolactone Membrane for the Segregated Co-Culture of Bone and Soft Tissue. Polymers. 2016; 8(6):221. https://doi.org/10.3390/polym8060221
Chicago/Turabian StylePuwanun, Sasima, Frazer J. Bye, Moira M. Ireland, Sheila MacNeil, Gwendolen C. Reilly, and Nicola H. Green. 2016. "Production and Characterization of a Novel, Electrospun, Tri-Layer Polycaprolactone Membrane for the Segregated Co-Culture of Bone and Soft Tissue" Polymers 8, no. 6: 221. https://doi.org/10.3390/polym8060221
APA StylePuwanun, S., Bye, F. J., Ireland, M. M., MacNeil, S., Reilly, G. C., & Green, N. H. (2016). Production and Characterization of a Novel, Electrospun, Tri-Layer Polycaprolactone Membrane for the Segregated Co-Culture of Bone and Soft Tissue. Polymers, 8(6), 221. https://doi.org/10.3390/polym8060221