Oxidized Xanthan Gum and Chitosan as Natural Adhesives for Cork
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oxidized Xanthan Gum
2.3. Oxidation Degree Determination
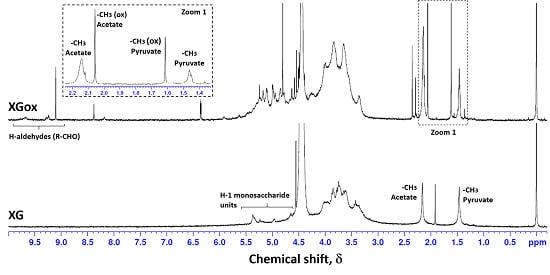
2.4. Nuclear Magnetic Resonance (NMR)
2.5. Preparation of Glues
2.6. Total Soluble Matter (TSM)
2.7. Tensile Strength Test
3. Results and Discussion
3.1. Xanthan Gum Oxidation
3.2. Total Soluble Matter
3.3. Tensile Strength
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| COSY | Correlation spectroscopy |
| CS | Chitosan |
| DA | Degree of acetylation |
| D2O | Deuterium oxide |
| HSQC | Heteronuclear single quantum correlation |
| HMBC | Heteronuclear multiple-bond correlation |
| MWCO | Molecular weight cut-off |
| NMR | Nuclear magnetic resonance |
| PFA | Perfluoroalkoxy |
| PUR | Polyurethane adhesive |
| TMS | Total soluble matter |
| TMSP | Trimethylsilyl propanoic acid |
| XG | Xanthan gum |
| XGox | Oxidized xanthan gum |
References
- Graça, J.; Santos, S. Suberin: A biopolyester of plants’ skin. Macromol. Biosci. 2007, 7, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J.; Easterling, K.E.; Ashby, M.F. The structure and mechanics of cork. Proc. R. Soc. A 1981, 377, 99–117. [Google Scholar] [CrossRef]
- Pereira, H. The rationale behind cork properties: A review of structure and chemistry. BioResource 2015, 10, 6207–6229. [Google Scholar] [CrossRef]
- Cordeiro, N.; Belgacem, M.N.; Gandini, A.; Pascoal Neto, C. Urethanes and polyurethanes from suberin 2: Synthesis and characterization. Ind. Crop. Prod. 1999, 10, 1–10. [Google Scholar] [CrossRef]
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef]
- Pereira, H. Chemical composition and variability of cork from quercus suber l. Wood Sci. Technol. 1988, 22, 211–218. [Google Scholar] [CrossRef]
- Barbosa, A.Q.; da Silva, L.F.M.; Abenojar, J.; del Real, J.C.; Paiva, R.M.M.; Öchsner, A. Kinetic analysis and characterization of an epoxy/cork adhesive. Thermochim. Acta 2015, 604, 52–60. [Google Scholar] [CrossRef]
- Barbosa, A.Q.; da Silva, L.F.M.; Öchsner, A.; Abenojar, J.; del Real, J.C. Influence of the size and amount of cork particles on the impact toughness of a structural adhesive. J. Adhes. 2012, 88, 452–470. [Google Scholar] [CrossRef]
- Imam, S.H.; Gordon, S.H.; Mao, L.; Chen, L. Environmentally friendly wood adhesive from a renewable plant polymer: Characteristics and optimization. Polym. Degrad. Stab. 2001, 73, 529–533. [Google Scholar] [CrossRef]
- Cunha, A.G.; Gandini, A. Turning polysaccharides into hydrophobic materials: A critical review. Part 1. Cellulose. Cellulose 2010, 17, 875–889. [Google Scholar] [CrossRef]
- Baumann, M.G.D.; Conner, A.H. Carbohydrate polymers as adhesives. In Handbook of Adhesive Technology, 2nd ed.; Pizzi, A., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 2003; pp. 375–379. [Google Scholar]
- Jansson, P.E.; Kenne, L.; Lindberg, B. Structure of the extracellular polysaccharide from xanthomonas campestris. Carbohyd. Res. 1975, 45, 275–282. [Google Scholar] [CrossRef]
- Soares, R.M.D.; Lima, A.M.F.; Oliveira, R.V.B.; Pires, A.T.N.; Soldi, V. Thermal degradation of biodegradable edible films based on xanthan and starches from different sources. Polym. Degrad. Stab. 2005, 90, 449–454. [Google Scholar] [CrossRef]
- Katzbauer, B. Properties and applications of xanthan gum. Polym. Degrad. Stab. 1998, 59, 81–84. [Google Scholar] [CrossRef]
- Tako, M.; Teruya, T.; Tamaki, Y.; Ohkawa, K. Co-gelation mechanism of xanthan and galactomannan. Colloid Polym. Sci. 2010, 288, 1161–1166. [Google Scholar] [CrossRef]
- Lopes, L.; Andrade, C.T.; Milas, M.; Rinaudo, M. Role of conformation and acetylation of xanthan on xanthan-guar interaction. Carbohydr. Polym. 1992, 17, 121–126. [Google Scholar] [CrossRef]
- Bryant, C.M.; McClements, D.J. Influence of xanthan gum on physical characteristics of heat-denatured whey protein solutions and gels. Food Hydrocoll. 2000, 14, 383–390. [Google Scholar] [CrossRef]
- Hemar, Y.; Tamehana, M.; Munro, P.A.; Singh, H. Viscosity, microstructure and phase behavior of aqueous mixtures of commercial milk protein products and xanthan gum. Food Hydrocoll. 2001, 15, 565–574. [Google Scholar] [CrossRef]
- Norström, E.; Fogelström, L.; Nordqvist, P.; Khabbaz, F.; Malmström, E. Gum dispersions as environmentally friendly wood adhesives. Ind. Crop. Prod. 2014, 52, 736–744. [Google Scholar] [CrossRef]
- Sloan, J.W.; HoFreiter, B.T.; Mellies, R.L.; Wolff, I.A. Properties of periodate oxidized starch. Ind. Eng. Chem. 1956, 48, 1165–1172. [Google Scholar] [CrossRef]
- Slager, J. Process for Periodate Oxidation of Polysaccharides. U.S. Patent 3,086,969 A, 23 April 1963. [Google Scholar]
- Li, H.; Wu, B.; Mu, C.; Lin, W. Concomitant degradation in periodate oxidation of carboxymethyl cellulose. Carbohydr. Polym. 2011, 84, 881–886. [Google Scholar] [CrossRef]
- Guo, J.; Ge, L.; Li, X.; Mu, C.; Li, D. Periodate oxidation of xanthan gum and its crosslinking effects on gelatin-based edible films. Food Hydrocoll. 2014, 39, 243–250. [Google Scholar] [CrossRef]
- Serrero, A.; Trombotto, S.; Bayon, Y.; Gravagna, P.; Montanari, S.; David, L. Polysaccharide-based adhesive for biomedical applications: Correlation between rheological behavior and adhesion. Biomacromolecules 2011, 12, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.G.; Gandini, A. Turning polysaccharides into hydrophobic materials: A critical review. Part 2. Hemicelluloses, chitin/chitosan, starch, pectin and alginates. Cellulose 2010, 17, 1045–1065. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.S.; Ramasamy, M.; Suresh, B. Chitosan nanoparticles as a new delivery system for the anti-alzheimer drug tacrine. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Son, J.H.; Kim, S.-K.; Weller, C.L.; Hanna, M.A. Properties of chitosan films as a function of pH and solvent type. J. Food Sci. 2006, 71, E119–E124. [Google Scholar] [CrossRef]
- Argin-Soysal, S.; Kofinas, P.; Lo, Y.M. Effect of complexation conditions on xanthan–chitosan polyelectrolyte complex gels. Food Hydrocoll. 2009, 23, 202–209. [Google Scholar] [CrossRef]
- Hoffmann, B.; Volkmer, E.; Kokott, A.; Augat, P.; Ohnmacht, M.; Sedlmayr, N.; Schieker, M.; Claes, L.; Mutschler, W.; Ziegler, G. Characterisation of a new bioadhesive system based on polysaccharides with the potential to be used as bone glue. J. Mater. Sci. Mater. Med. 2009, 20, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Serrero, A.; Trombotto, S.; Cassagnau, P.; Bayon, Y.; Gravagna, P.; Montanari, S.; David, L. Polysaccharide gels based on chitosan and modified starch: Structural characterization and linear viscoelastic behavior. Biomacromolecules 2010, 11, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Chen, T.; Kumar, G.; Vesnovsky, O.; Topoleski, L.D.; Payne, G.F. Chitosan based water-resistant adhesive. Analogy to mussel glue. Biomacromolecules 2000, 1, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Michaud, P.; Petit, E.; de Baynast, H.; Grédiac, M.; Mathias, J.-D. Development of a chitosan-based adhesive. Application to wood bonding. J. Appl. Polym. Sci. 2013, 127, 5014–5021. [Google Scholar] [CrossRef]
- Umemura, K.; Inoue, A.; Kawai, S. Development of new natural polymer-based wood adhesives І: Dry bond strength and water resistance of konjac glucomannan, chitosan and their composites. J. Wood Sci. 2003, 49, 221–226. [Google Scholar] [CrossRef]
- Zhao, H.; Heindel, N.D. Determination of degree of substitution of formyl groups in polyaldehyde dextran by the hydroxylamine hydrochloride method. Pharm. Res. 1991, 8, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, N.W.H.; Mashimba, E.N.M. Proton and carbon-13 NMR studies on xanthan derivatives. Carbohydr. Polym. 1992, 17, 127–136. [Google Scholar] [CrossRef]
- Rinaudo, M.; Milas, M.; Lambert, F.; Vincendon, M. Proton and carbon-13 NMR investigation of xanthan gum. Macromolecules 1983, 16, 816–819. [Google Scholar] [CrossRef]
- Horton, D.; Mols, O.; Walaszek, Z.; Wernau, W.C. Structural and biosynthetic-studies on xanthan by 13C-NMR spectroscopy. Carbohydr. Res. 1985, 141, 340–346. [Google Scholar] [CrossRef]
- Roslund, M.U.; Tahtinen, P.; Niemitz, M.; Sjhohn, R. Complete assignments of the 1H and 13C chemical shifts and JH,H coupling constants in NMR spectra of d-glucopyranose and all d-glucopyranosyl-d-glucopyranosides. Carbohydr. Res. 2008, 343, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, R.; de Waard, P.; Schols, H.A.; Siika-aho, M.; Voragen, A.G.J. Methylobacterium sp isolated from a finnish paper machine produces highly pyruvated galactan exopolysaccharide. Carbohydr. Res. 2003, 338, 1851–1859. [Google Scholar] [CrossRef]
- Kuo, M.S.; Mort, A.J.; Dell, A. Identification and location of l-glycerate, an unusual acyl substituent in gellan gum. Carbohydr. Res. 1986, 156, 173–187. [Google Scholar] [CrossRef]
- Garcı́a-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Helleur, R.J. Characterization of the saccharide composition of heteropolysaccharides by pyrolysis capillary gas-chromatography mass-spectrometry. J. Anal. Appl. Pyrol. 1987, 11, 297–311. [Google Scholar] [CrossRef]
- Tait, M.I.; Sutherland, I.W.; Clarke-Sturman, A.J. Effect of growth conditions on the production, composition and viscosity of xanthomonas campestris exopolysaccharide. Microbiology 1986, 132, 1483–1492. [Google Scholar] [CrossRef]
- Holzwarth, G.; Ogletree, J. Pyruvate-free xanthan. Carbohydr. Res. 1979, 76, 277–280. [Google Scholar] [CrossRef]
- Gerken, T.A.; Gupta, R.; Jentoft, N. A novel-approach for chemically deglycosylating O-linked glycoproteins. The deglycosylation of submaxillary and respiratory mucins. Biochemistry 1992, 31, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.C.; Kim, Y.S. Alkali-catalyzed β-elimination of periodate-oxidized glycans: A novel method of chemical deglycosylation of mucin gene products in paraffin embedded sections. Glycoconj. J. 2000, 17, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Veelaert, S.; de Wit, D.; Gotlieb, K.F.; Verhé, R. Chemical and physical transitions of periodate oxidized potato starch in water. Carbohydr. Polym. 1997, 33, 153–162. [Google Scholar] [CrossRef]
- Kim, U.J.; Kuga, S.; Wada, M.; Okano, T.; Kondo, T. Periodate oxidation of crystalline cellulose. Biomacromolecules 2000, 1, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Haaksman, I.K.; Besemer, A.C.; Jetten, J.M.; Timmermans, J.W.; Slaghek, T.M. The oxidation of the aldehyde groups in dialdehyde starch. Starch/Stärke 2006, 58, 616–622. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Potthast, A.; Christensen, B.E. Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr. Res. 2010, 345, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Dryhurst, G.; Belcher, R.; Anderson, D.M.W. Periodate Oxidation of Diol and Other Functional Groups: Analytical and Structural Applications; Pergamon Press: Oxford, UK, 1970; Volume 2. [Google Scholar]
- Perlin, A.S. Glycol-cleavage oxidation. Adv. Carbohydr. Chem. Biochem. 2006, 60, 183–250. [Google Scholar] [PubMed]
- Mati-Baouche, N.; Elchinger, P.-H.; de Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar] [CrossRef]






| δ (ppm) | Assignment | |
|---|---|---|
| 1H | 13C | |
| 1.458 | 27.615 | CH3 of pyruvated mannose (E) |
| 1.615 | 26.266 | CH3 of oxidized pyruvated mannose (E) |
| 2.071 | 23.605 | CH3 of oxidized O-acetylated mannose (C) |
| 2.134 | 23.423 | CH3 of O-acetylated mannose (C) |
| 3.654 | 60.740 | CH2 (C-6) of non-pyruvated mannose (oxidized) (E4) |
| 4.482 | 64.340 | CH2 (C-6) of mannose after β-elimination (E2) |
| 4.598 | 64.340 | CH2 (C-6) of mannose after β -elimination (E2) |
| 6.354 | 126.686 | CH ethylenic carbon of mannose after β-elimination (E2) |
| – | 152.060 | qC ethylenic carbon of mannose after β-elimination (E2) |
| 9.103 | 191.210 | HC=O of mannose after β-elimination (E2) |
| 9.223 | 194.137 | HC=O of non-pyruvated mannose (oxidized) (E4) |
| Adhesive | TSM (%) | Observations |
|---|---|---|
| XG | 100 | Film dissolves completely, forming a solution with low viscosity. |
| XGox | 78 ± 5 | Film divides into smaller pieces and dissolves partially; no swelling observed in the surviving film pieces. |
| CS | 100 | Film dissolves completely, forming a highly viscous solution. |
| XG:CS | 31 ± 2 | Film dissolves partially, but maintains its integrity; swelling is visible, especially in the borders. |
| XGox:CS | 20 ± 2 | Film dissolves partially, but maintains its integrity; no swelling is visible. |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, D.; Gonçalves, C.; Vale, I.; Bastos, M.M.S.M.; Magalhães, F.D. Oxidized Xanthan Gum and Chitosan as Natural Adhesives for Cork. Polymers 2016, 8, 259. https://doi.org/10.3390/polym8070259
Paiva D, Gonçalves C, Vale I, Bastos MMSM, Magalhães FD. Oxidized Xanthan Gum and Chitosan as Natural Adhesives for Cork. Polymers. 2016; 8(7):259. https://doi.org/10.3390/polym8070259
Chicago/Turabian StylePaiva, Diana, Carolina Gonçalves, Isabel Vale, Margarida M. S. M. Bastos, and Fernão D. Magalhães. 2016. "Oxidized Xanthan Gum and Chitosan as Natural Adhesives for Cork" Polymers 8, no. 7: 259. https://doi.org/10.3390/polym8070259
APA StylePaiva, D., Gonçalves, C., Vale, I., Bastos, M. M. S. M., & Magalhães, F. D. (2016). Oxidized Xanthan Gum and Chitosan as Natural Adhesives for Cork. Polymers, 8(7), 259. https://doi.org/10.3390/polym8070259