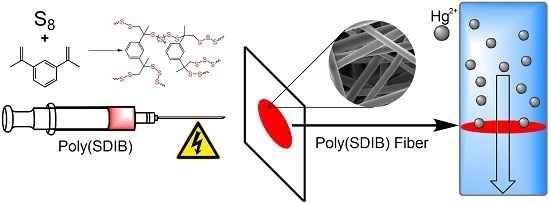
Rapid Mercury(II) Removal by Electrospun Sulfur Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Poly(SDIB)
2.2. Analytical Methods
2.3. Electrospinning
2.4. Metal Ion Sorption
3. Results
3.1. Electrospinning
3.2. Metal Ion Sorption Studies
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lubick, N.; Malakoff, D. With pact’s completion, the real work begins. Science 2013, 341, 1443–1445. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W. Mercury: Major issues in environmental health. Environ. Health Perspect. 1993, 100, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kim, D.; Dionysiou, D.D.; Sorial, G.A.; Timberlake, D. Sources and remediation for mercury contamination in aquatic systems—A literature review. Environ. Pollut. 2004, 131, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical precipitation of heavy metals from acid mine drainage. Water Res. 2002, 36, 4757–4764. [Google Scholar] [CrossRef]
- Chiarle, S. Mercury removal from water by ion exchange resins adsorption. Water Res. 2000, 34, 2971–2978. [Google Scholar] [CrossRef]
- Misaelides, P.; Godelitsas, A. Removal of heavy metals from aqueous solutions using pretreated natural zeolitic materials: The case of mercury(II). Toxicol. Environ. Chem. 1995, 51, 21–29. [Google Scholar] [CrossRef]
- Athanasopoulos, P.S.; Jacob, W.; Neumann, S.; Kutsch, M.; Wolters, D.; Tan, E.K.; Bichler, Z.; Heumann, R. Identification of protein phosphatase 2A as an interacting protein of leucine-rich repeat kinase 2. Biol. Chem. 2016, 397, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, C.; Kadirvelu, K. Uptake of mercury(II) from wastewater by activated carbon from an unwanted agricultural solid by-product: Coirpith. Carbon 1999, 37, 79–84. [Google Scholar] [CrossRef]
- Uludag, Y.; Özbelge, H.Ö.; Yilmaz, L. Removal of mercury from aqueous solutions via polymer-enhanced ultrafiltration. J. Memb. Sci. 1997, 129, 93–99. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, A.; Arya, R.K. Removal of mercury(II) from aqueous solution: A review of recent Work. Sep. Sci. Technol. 2014, 50, 1310–1320. [Google Scholar] [CrossRef]
- Du, Q.; Wu, J.; Yang, H. Pt@Nb-TiO2 catalyst membranes fabricated by electrospinning and atomic layer deposition. ACS Catal. 2014, 4, 144–151. [Google Scholar] [CrossRef]
- Miao, Y.; Fan, W.; Chen, D.; Liu, T. High-performance supercapacitors based on hollow polyaniline nano fibers by electrospinning. ACS Appl. Mater. Interfaces 2013, 5, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xu, X.; Liu, Q.; Cheng, J.; Yuan, X.; Wu, L.; Wan, Y. Electrospun poly(vinyl alcohol)/glucose oxidase biocomposite membranes for biosensor applications. React. Funct. Polym. 2006, 66, 1559–1564. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Yanilmaz, M.; Lu, Y.; Dirican, M.; Fu, K.; Zhang, X. Nanoparticle-on-nanofiber hybrid membrane separators for lithium-ion batteries via combining electrospraying and electrospinning techniques. J. Memb. Sci. 2014, 456, 57–65. [Google Scholar] [CrossRef]
- Huang, Y.; Miao, Y.E.; Liu, T. Electrospun fibrous membranes for efficient heavy metal removal. J. Appl. Polym. Sci. 2014, 131, 1–12. [Google Scholar] [CrossRef]
- Wanjale, S.; Birajdar, M.; Jog, J.; Neppalli, R.; Causin, V.; Karger-Kocsis, J.; Lee, J.; Panzade, P. Surface tailored PS/TiO2 composite nanofiber membrane for copper removal from water. J. Colloid Interface Sci. 2016, 469, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Li, F.; Gu, Q.; Liang, C.; Chen, J. Heavy metal-contaminated groundwater treatment by a novel nanofiber membrane. Desalination 2008, 223, 349–360. [Google Scholar] [CrossRef]
- Thielke, M.W.; Secker, C.; Schlaad, H.; Theato, P. Electrospinning of crystallizable polypeptoid fibers. Macromol. Rapid Commun. 2015, 37, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Crockett, M.P.; Evans, A.M.; Worthington, M.J.H.; Albuquerque, I.S.; Slattery, A.D.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Bernardes, G.J.L.; Chalker, J.M. Sulfur-limonene polysulfide: A material synthesized entirely from industrial by-products and its use in removing toxic metals from water and soil. Angew. Chem. Int. Ed. 2015, 55, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Hasell, T.; Parker, D.J.; Jones, H.A.; McAllister, T.; Howdle, S.M. Porous inverse vulcanised polymers for mercury capture. Chem. Commun. 2016, 52, 5383–5386. [Google Scholar] [CrossRef] [PubMed]
- Dirlam, P.T.; Simmonds, A.G.; Kleine, T.S.; Nguyen, N.A.; Anderson, L.E.; Klever, A.O.; Florian, A.; Costanzo, P.J.; Theato, P.; Mackay, M.E.; et al. Inverse vulcanization of elemental sulfur with 1,4-diphenylbutadiyne for cathode materials in Li–S batteries. RSC Adv. 2015, 5, 24718–24722. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, A.G.; Griebel, J.J.; Park, J.; Kim, K.R.; Chung, W.J.; Oleshko, V.P.; Kim, J.; Kim, E.T.; Glass, R.S.; Soles, C.L.; et al. Inverse vulcanization of elemental sulfur to prepare polymeric electrode materials for Li-S batteries. ACS Macro Lett. 2014, 3, 229–232. [Google Scholar] [CrossRef]
- Griebel, J.J.; Namnabat, S.; Kim, E.T.; Himmelhuber, R.; Moronta, D.H.; Chung, W.J.; Simmonds, A.G.; Kim, K.J.; van der Laan, J.; Nguyen, N.A.; et al. New infrared transmitting material via inverse vulcanization of elemental sulfur to prepare high refractive index polymers. Adv. Mater. 2014, 26, 3014–3018. [Google Scholar] [CrossRef] [PubMed]
- Griebel, J.J.; Nguyen, N.A.; Namnabat, S.; Anderson, L.E.; Glass, R.S.; Norwood, R.A.; Mackay, M.E.; Char, K.; Pyun, J. Dynamic covalent polymers via inverse vulcanization of elemental sulfur for healable infrared optical materials. ACS Macro Lett. 2015, 4, 862–866. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Ke, F.; Qiu, L.G.; Yuan, Y.P.; Peng, F.M.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2011, 196, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Fryxell, G.E.; Um, W.; Parker, K.; Mattigod, S.V.; Skaggs, R. Sulfur-functionalized mesoporous carbon. Adv. Funct. Mater. 2007, 17, 2897–2901. [Google Scholar] [CrossRef]
- Yee, K.K.; Reimer, N.; Liu, J.; Cheng, S.Y.; Yiu, S.M.; Weber, J.; Stock, N.; Xu, Z. Effective mercury sorption by thiol-laced metal-organic frameworks: In strong acid and the vapor phase. J. Am. Chem. Soc. 2013, 135, 7795–7798. [Google Scholar] [CrossRef] [PubMed]
- Cossio, M.L.T.; Giesen, L.F.; Araya, G.; Pérez-Cotapos, M.L.S.; Vergara, R.L.; Manca, M.; Tohme, R.A.; Holmberg, S.D.; Bressmann, T.; Lirio, D.R.; et al. Porus semiconducting gels and arogels from chalcogenide clusters. Science 2007, 317, 490–493. [Google Scholar]
- Liu, J.; Feng, X.; Fryxell, G.E.; Wang, L.-Q.; Kim, A.Y.; Gong, M. Hybrid mesoporous materials with functionalized monolayers. Adv. Mater. 1998, 10, 161–165. [Google Scholar] [CrossRef]
- Feng, X. Functionalized monolayers on ordered mesoporous supports. Science 1997, 276, 923–926. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Y.; Ma, D.; Shi, Z.; Ma, S. Mercury nano-trap for effective and efficient removal of mercury(II) from aqueous solution. Nat. Commun. 2014, 5, 5537. [Google Scholar] [CrossRef] [PubMed]




| PMMA concentration (wt %) | Poly(SDIB) content of the fiber (wt %) | SEM-Resulting appearance |
|---|---|---|
| 3 | 85.5 | Few fibers, predominantly electrosprayed particles |
| 4 | 81.5 | heavily beaded fibers |
| 5 | 77.9 | heavily beaded fibers |
| 6 | 74.6 | beaded fibers |
| 7 | 71.6 | beaded fibers |
| 8 | 68.8 | predominantly smooth fibers, few beads |
| 9 1 | 66.2 1 | fibers 1 |
| Polymer | Concentration | SFE (mN/m) |
|---|---|---|
| PMMA | 10 wt % | 38.8 ± 0.9 |
| Poly(SDIB) | 10 wt % | 27.4 ± 1.8 |
| Blend | 7.65 wt % (PMMA) + 15 wt % (Poly(SDIB)) | 30.1 ± 0.7 |
| Salt | Metal ion | Adsorbed percentage (%) |
|---|---|---|
| Cd(NO3)2·4 H2O | Cd2+ | 12.9 |
| Co(NO3)2·6 H2O | Co2+ | 12.5 |
| Cu(NO3)2·3 H2O | Cu2+ | 12.2 |
| FeNO3)2·4 H2O | Fe3+ | 18.9 |
| Pb(NO3)2 | Pb2+ | 13.6 |
| Zn(NO3)2·4 H2O | Zn2+ | 16.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thielke, M.W.; Bultema, L.A.; Brauer, D.D.; Richter, B.; Fischer, M.; Theato, P. Rapid Mercury(II) Removal by Electrospun Sulfur Copolymers. Polymers 2016, 8, 266. https://doi.org/10.3390/polym8070266
Thielke MW, Bultema LA, Brauer DD, Richter B, Fischer M, Theato P. Rapid Mercury(II) Removal by Electrospun Sulfur Copolymers. Polymers. 2016; 8(7):266. https://doi.org/10.3390/polym8070266
Chicago/Turabian StyleThielke, Michael W., Lindsey A. Bultema, Daniel D. Brauer, Bernadette Richter, Markus Fischer, and Patrick Theato. 2016. "Rapid Mercury(II) Removal by Electrospun Sulfur Copolymers" Polymers 8, no. 7: 266. https://doi.org/10.3390/polym8070266
APA StyleThielke, M. W., Bultema, L. A., Brauer, D. D., Richter, B., Fischer, M., & Theato, P. (2016). Rapid Mercury(II) Removal by Electrospun Sulfur Copolymers. Polymers, 8(7), 266. https://doi.org/10.3390/polym8070266