3.1. Treatment of the Nomex®/Cotton Fabric by BTCA
Polycarboxylic acids exemplified by BTCA have long been used as crosslinking agents for cotton and wood cellulose [
22,
23]. They were known as formaldehyde-free wrinkle resistant finishing agents for cotton. Polycarboxylic acids were first reported as flame retardants for cotton carpets in 2002 [
24]. In our previous research, we investigated the applications of different polycarboxylic acids, such as BTCA, citric acid and succinic acid, to flame retardant finishing of cotton fleece [
25,
26,
27,
28]. It was discovered that BTCA and other polycarboxylic acids were able to reduce the flammability of cotton fleece from “Class 3” (high flammability) to “Class 1” (normal flammability) according to the U.S. government regulation 16 CFR 1610.
In this research, the effects of BTCA on the peak heat release rate (PHRR), heat release capacity (HRC), percent char yield and vertical burning flammability of cotton, Nomex
® and the 65/35 Nomex
®/cotton blend fabrics are evaluated. The PHRR of 100% cotton is 274 w/g (
Figure 1), and it becomes 89 w/g for the cotton in the Nomex
®/cotton blend (
Figure 2) because the blend contains approximately 35% cotton. The PHRR of cotton in the Nomex
®/cotton blend treated with 3% BTCA is 67 w/g, representing 25% reduction, whereas the temperature at PHRR (
TPHRR) changes very little (
Figure 2). The BTCA treatment has no effect on both PHRR and
TPHRR of Nomex
® in the blend, as shown in
Figure 2.
Presented in
Table 1 is the PHRR, HRC and char yield of cotton on the Nomex
®/cotton blend treated with BTCA with different concentrations. The decrease in PHRR and HRC of cotton was in the ranges of 21%–25% and 19%–22%, respectively, which appears to be independent of BTCA concentration. The char yield of the untreated blend fabric was 31.2%, which is mostly from the decomposition of Nomex
® in the blend. The data show that the BTCA treatment caused little increase in char yield (
Table 1). The LOI of the treated blend fabric becomes marginally higher than that of the untreated blend fabric. All the fabric samples fail the vertical flammability test (
Table 2).
3.2. Treatment of the Nomex®/Cotton Fabric by BTCA and HFPO
The 65/35 Nomex
®/cotton blend fabric is treated with 3% BTCA in combination with HFPO at concentration ranging from 6% to 14%, cured at 170 °C for 3 min and finally subjected to one home washing cycle. Presented in
Table 3 is the phosphorus concentration of the fabric thus treated before and after the washing procedure. Shown in
Figure 3 is the percent phosphorus retentions, which is the percentage of the applied phosphorus bound to the blend fabric with respect to their prewash values. The data demonstrate that as the HFPO concentration is increased from 6% to 14%, the phosphorus concentration on the fabric after wash increases from 0.37% to 0.64% (
Table 3) whereas the percent phosphorus retention decreases from 80% to 62%, respectively (
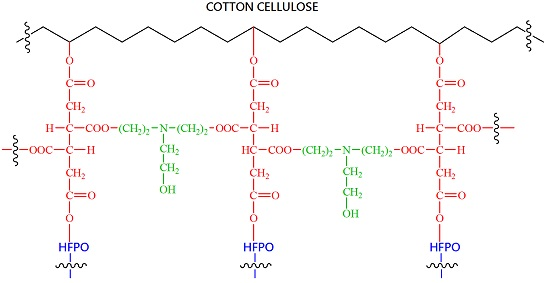
Figure 3). Both HFPO and cotton cellulose are hydroxy-functional compounds. They compete to esterify BTCA on the fabric as shown in
Scheme 2. At a constant BTCA concentration, increasing HFPO concentration causes the esterification of HFPO/BTCA to increase and consequently esterification of cotton/BTCA to decrease. As a result, percent of the HFPO bound to cotton decreases in spite of the increase in total HFPO bound to cotton as shown in
Figure 3 and
Table 3, respectively. This is because the bonding of HFPO to cotton requires BTCA to esterify both cotton and HFPO. Therefore, increasing HFPO concentration increases the phosphorus bound to cotton due to an increase in HFPO/BTCA bonding, but it also reduces the percentage of HFPO bound to cotton because it reduces BTCA/cotton bonding. The data in
Figure 3 show that 80% of the HFPO was bound to cotton at 6% HFPO. The phosphorus retention decreases to 64% at 12% HFPO. Increasing the HFPO concentration further to 14% reduces the phosphorus retention to 62%.
The calcium concentration of the treated blend fabric was also presented in
Table 3. Calcium is an element having significant impact on the flame retarding performance of phosphorus-based flame retardants on cotton. Previously, we found that calcium was bound to the cotton treated with HFPO/BTCA after multiple launderings due to the formation of insoluble calcium salts [
20]. Such calcium salts is detrimental to the flame retarding properties of phosphorus-based flame retardants on cotton and cotton blends [
20,
21]. In this research, calcium is detected on the Nomex/cotton blend fabric treated with HFPO/BTCA. Calcium concentration decreases from 0.075% to 0.065% as the HFPO concentration for the treatment is increased from 6% to 14% (
Table 3). The declining calcium concentrations at higher HFPO concentrations shown in
Table 3 is due to increasing esterification of BTCA/HFPO at higher HFPO concentration, which reduces the quantity of free carboxy of BTCA on cotton available for forming calcium salt.
Presented in
Figure 4 is the stiffness of the blend fabric treated with the combination of 3% BTCA and HFPO at different concentrations, cured at 170 °C for 3 min and finally subjected to one washing cycle. Because HFPO has two hydroxyl groups in its molecule whereas BTCA has four carboxyl groups, it is most likely that the reactions between HFPO and BTCA form a crosslinked polymeric network on the Nomex
®/cotton blend fabric. The fabric stiffness increases significantly when the HFPO concentration is increased to 10%–14% (
Figure 4), which supports the hypothesis that the reactions of BTCA and HFPO forms crosslinked polymeric networks.
Figure 4 also shows that formation of such crosslinked polymeric network is dependent on the HFPO/BTCA ratio. The same phenomenon was observed on the nylon/cotton blend fabrics treated with HFPO and DMDHEU in our previous research [
29].
Presented in
Table 4 are HRC, PHRR, total heat release (THR), temperature at PHRR (
TPHRR) and char yield of the Nomex
®/cotton blend fabric treated with 3% BTCA and HFPO at different concentrations. The HRR vs. temperature curves of the Nomex
®/cotton blend without treatment and that treated with 3% BTCA and HFPO (6% and 14%) are shown in
Figure 5. Drastic changes take place in the HRR vs. temperature curves when the blend fabric is treated with HFPO/BTCA. Without treatment, the HRR of cotton reaches its peak (90 w/g) at 371 °C. When the blend fabric is treated with 3% BTCA, the HRR peak (67 w/g) appears at 369 °C (
Figure 2). When 6% HFPO is added to the treatment, the HRR peak decreases to 57 w/g at 315 °C (
Figure 5). The char yield was 31.2% and 31.4% for the untreated fabric and that treated by 3% BTCA, respectively (
Table 1). The char yield increases considerably to 41.9% when 6% HFPO is added for the treatment (
Table 4). HRC, PHRR, total heat release (THR) and
TPHRR all decrease and char yield increases as the HFPO concentration is increased from 6% to 14% (
Figure 5 and
Table 4). The data also demonstrate that the changes in heat release properties and char yield are dependent on HFPO concentration (
Table 4). Those significant changes are obviously due to the presence of phosphorus on the fabric, which changes cellulose degradation process to promote dehydration and also lowers the degradation temperature.
Figure 5 also shows that the HFPO/BTCA system reduces the PHRR of poly(meta-aramide) on the blend.
The LOI and vertical burning flammability data of the Nomex
®/cotton blend thus treated are presented in
Table 5. Apparently, the HFPO/BTCA system is effective in imparting flame retarding properties to the blend fabric. The use of 6% HFPO in the flame retardant system drastically reduces the vertical burning char length from >300 mm (total burning) to 51 mm and increases LOI from 22.8% to 27.6%. The char length decreases and LOI increases significantly as the HFPO concentration increases from 6% to 14% (
Table 5).
3.3. Treatment of the Nomex®/Cotton Fabric by BTCA, HFPO, and TEA
To study the chemical reactions of TEA with HFPO/BTCA, the fabric is treated with 12% HFPO, 3% BTCA and TEA with concentration ranging from 1% to 4%. Since TEA is a base, the traditional catalyst (NaH2PO2) is replaced by the acidic H3PO2. The final pH of all HFPO/BTCA/TEA solutions is adjusted to ~2.8 by adding NaOH or HCl depending on the quantity of TEA added to a solution. The fabric thus treated is cured at 170 °C for 3 min and finally subjected one regular home laundering cycle. To simulate a washing condition using water with high hardness, the fabric thus treated is subjected to a second washing cycle, in which 0.01% Ca(NO3)2 is added to the laundering solution to raise water hardness.
The phosphorus concentrations before wash, after one wash and after two washes are shown as
P0,
P1 and
P2, respectively, in
Table 6. The phosphorus retentions (%) after one and two washes shown in
Figure 6 are defined as (
P1/
P0) × 100% and (
P2/
P0) × 100%, respectively.
Table 6 shows that the phosphorus concentration is in the vicinity of 0.91% for all the treated fabric samples before washing. When the treated fabric is subjected to a regular wash, phosphorus concentration (
P1) on the treated fabric increases from 0.61 to the maximum (0.72) as the TEA concentration is raised from 0% to 2%. After the second washing procedure, the fabric treated without TEA retains 74% of the phosphorus after the first wash whereas that treated with 1% TEA retained 89% of the phosphorus after the first wash (
Table 6). The fabric treated using 3% TEA has the highest phosphorus concentration of 0.69%, which reveals that the fabric retained 99% of the phosphorus after the first wash. When the TEA concentration is increased to 4%, the phosphorus concentration decreases to 0.67% (
Table 6).
Figure 6 shows that after the second wash, phosphorus retention for the fabric treated without TEA is 50.0%, whereas that for the fabric treated with 3% TEA was 75.8%. Further increasing TEA to 4% slightly reduces the phosphorus retention to 73.6%.
The data presented here indicate that adding TEA as an additive to the HFPO/BTCA system significantly increases phosphorus retention of the treated fabric subjected to launderings. TEA has three hydroxy groups in its molecule and is able to esterify BTCA to form a HFPO/BTCA/TEA/cotton crosslinked network as shown in
Scheme 3, thus improving hydrolysis-resistance of the HFPO bound onto the blend. The data also demonstrate that the TEA concentration has an optimum range (~3%). Since TEA, HFPO and cotton are all hydroxy-functional compounds, a further increase in TEA concentration reduces the esterification between cotton and BTCA and that between HFPO and BTCA, thus reducing the bonding of HFPO to cotton and lowering the phosphorus retention.
Presented in
Figure 7 are the calcium concentrations of the Nomex
®/cotton blend fabric treated with 12.0% HFPO, 3.0% BTCA, and TEA at concentration ranging from 1% to 4% and subjected to two washing cycles against TEA concentration. The calcium concentration on the treated fabric after the first washing cycles decreases significantly as the TEA concentration is increased from 0% to 4% (
Figure 7). Esterification of BTCA by TEA reduces the concentration of free carboxy group on the fabric and consequently reduces formation of calcium salt. Adding Ca(NO
3)
2 in the second washing procedure leads to more calcium salt formation of the fabric. Consequently, TEA shows a more profound effect on the reduction of the calcium salt on the fabric (
Figure 7). The calcium concentration of the fabric treated with HFPO/BTCA without TEA is 0.118% after the second wash, whereas it drastically decreases to 0.054% when 4% TEA is used (
Figure 7).
Presented in
Figure 8a–d are the HRC, PHRR, THR and char yield of the Nomex
®/cotton fabric treated with 12% HFPO, 3% BTCA and TEA at different concentrations, cured and subjected to the two washing procedures as described above. The PHRR of the treated fabric without TEA is 61 w/g. It decreases to 59 w/g with 1% TEA added for the treatment, and it decreases further to 53 w/g with 3% TEA. The HRC and THR vs. TEA concentration curves follow the same trend (
Figure 8). The char yield of the fabric treated without TEA is 44.7%. It increases from 46.4% to the maximum of 48.9% when the TEA concentration is increased from 1% to 3%, respectively (
Figure 8). This phenomenon is consistent with the data presented in
Table 6. The phosphorus concentration of the fabric treated with 12% HFPO and 3% BTCA and subjected two washing cycles reaches its maximum when TEA concentration is 3%. The data presented here clearly demonstrate the effectiveness of TEA for reducing PHRR and increasing the char formation for the thermal degradation of the Nomex
®/cotton blend fabric treated with HFPO/BTCA/TEA.
Presented in
Table 7 are the LOI and vertical burning char length of the Nomex
®/cotton fabric treated with 12% HFPO, 3% BTCA and TEA at different concentrations, cured at 170 °C for 3 min and subjected to one regular washing cycle followed by a second wash with 0.01% Ca(NO
3)
2 added. The fabric treated using 3% TEA has the highest LOI (31.6%), which is two units higher than that of the fabric treated without TEA (29.6%). The char length of the fabric treated using TEA (36–39 mm) is also significantly lower than that of the fabric treated without TEA (45 mm). The data provide convincing evidence to prove the effectiveness of TEA for improving the fire performance of the HFPO/BTCA system on the blend fabric.
In our previous research [
21], the same Nomex
®/cotton fabric was treated with 24% HFPO, 8% BTCA, 2.5% H
3PO
2 and TEA with concentration ranging from 1% to 8%. Without laundering, all the treated Nomex/cotton fabric samples have the same concentrations of HFPO and H
3PO
2, both contain phosphorus, thus having the same phosphorus concentration, but they contain different TEA concentrations. The LOI (%) of the blend fabric increases from 37.2 to 40.6 as the TEA concentration increases from 0% to 8% [
21]. Thus, the LOI data demonstrate that the use of TEA serves another function. TEA improves flame retardant performance of the treated Nomex
®/cotton fabric by providing synergistic nitrogen.