Toxic Combustion Product Yields as a Function of Equivalence Ratio and Flame Retardants in Under-Ventilated Fires: Bench-Large-Scale Comparisons
Abstract
:1. Introduction
1.1. Combustion Conditions, Toxic Product Yields and Equivalence Ratios in Compartment Fires and Bench-Scale Tests
- Ability to reproduce a range of flaming set equivalence ratios over the range Φ ~ 0.5–3, which includes under-ventilated fires;
- Ability to provide yield data as a function of fuel mass loss;
- Ability to provide a hot effluent plume or upper layer over the range 300–850 °C;
- Ideally, to provide control of oxygen concentration in the air entering the combustion zone;
- Demonstrated ability to produce effluent yields comparable with the average and ranges of variability of yields obtained in compartment fires for a variety of fuels under equivalent combustion conditions across the Φ range.
1.2. Challenges with Measurement of Equivalence Ratios and Yields in Compartment Fires
1.3. Comparing Compartment Fire and Bench-Scale Data
2. Materials and Methods
2.1. Compartment Fire Experiments
2.2. Steady State Tube Furnace Experiments
2.3. Data Comparisons
3. Results
3.1. Results from Compartment Fire and SSTF Experiments on Six Materials
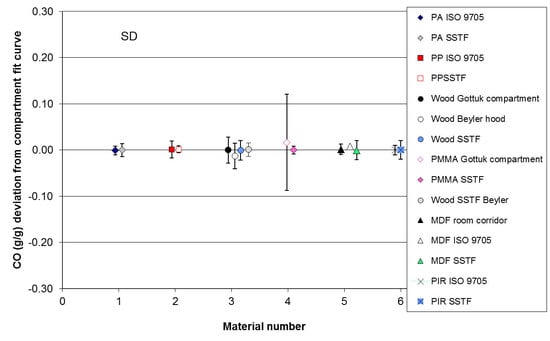
3.2. Results for Uncertainty and Accuracy Analysis off Compartment Fire and SSTF Data
3.3. HCN and Other Products of Inefficient Combustion
4. Discussion
4.1. Comparison of CO Yields as a Function of Φ between Compartment Fires and the SSTF
4.2. Effects of Flame Retardants on the Relationship between Φ and Yields of CO and HCN
4.3. Implications of Results for Toxic Hazards in Fires
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Purser, D.A.; Fardell, P.; Rowley, J.; Vollam, S.; Bridgeman, B.; Ness, E.M. An improved tube furnace method for the generation and measurement of toxic combustion products under a wide range of fire conditions. In Proceedings of the Flame Retardants Conference, London, UK, January 1994; Interscience Communications: London, UK, 1994; pp. 263–274. [Google Scholar]
- ISO TS 19700:2016. Controlled equivalence ratio method for the determination of hazardous components of fire effluents–the steady state tube furnace.
- Purser, D.A. Combustion toxicity. In SFPE Handbook of Fire Protection Engineering, 5th ed.; Hurley, M.J., Gottuk, D., Hall, J.R., Harada, K., Kuligowski, E., Puchovsky, M., Torero, J., Watts, J.M., Wieczorek, C., Eds.; Springer: New York, NY, USA, 2016; pp. 2207–2307. [Google Scholar] [CrossRef]
- Khan, M.M.; Tewarson, A.; Chaos, M. Combustion characteristics of materials and generation of fire products. In SFPE Handbook of Fire Protection Engineering, 5th ed.; Hurley, M.J., Gottuk, D., Hall, J.R., Harada, K., Kuligowski, E., Puchovsky, M., Torero, J., Watts, J.M., Wieczorek, C., Eds.; Springer: New York, NY, USA, 2016; pp. 1143–1231. [Google Scholar] [CrossRef]
- Purser, D.A. Fire types and combustion products. In Toxicity, Survival and Health Hazards of Combustion Products; Purser, D.A., Maynard, R.L., Wakefield, J., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 13–50. [Google Scholar]
- Purser, D.A. Estimating yields and quantities of mass releases of toxic products in from fires. In Toxicity, Survival and Health Hazards of Combustion Products; Purser, D.A., Maynard, R.L., Wakefield, J., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 53–76. [Google Scholar]
- Purser, D.A.; Purser, J.A. HCN yields and fate of fuel nitrogen for materials under different combustion conditions in the ISO 19700 tube furnace. In Fire Safety Science, Proceedings of the Ninth International Symposium, Karlsruhe, Germany, 21–26 September 2008; Karlsson, B., Ed.; International Association for Fire Safety Science: Greenwich, UK, 2008; pp. 1117–1128. [Google Scholar] [CrossRef]
- Purser, D.A.; Stec, A.A.; Hull, T.R. Effects of material and fire conditions on toxic product yields. In Fire Toxicity; Stec, A., Hull, R., Eds.; Woodhead: Cambridge, UK, 2010; pp. 515–540. [Google Scholar]
- Gottuk, D.T.; Lattimer, B.Y. Effect of combustion conditions on species production. In SFPE Handbook of Fire Protection Engineering, 5th ed.; Hurley, M.J., Gottuk, D., Hall, J.R., Harada, K., Kuligowski, E., Puchovsky, M., Torero, J., Watts, J.M., Wieczorek, C.T., Eds.; Springer: New York, NY, USA, 2016; pp. 486–528. [Google Scholar]
- Pitts, W.M. The global equivalence ratio concept and the formation mechanisms of carbon monoxide in fires. Prog. Energy Combust. Sci. 1995, 21, 197–237. [Google Scholar] [CrossRef]
- Purser, D.A.; Purser, J.A. The Potential for Including Fire Chemistry and Toxicity in Fire Safety Engineering; Project Report 202804; Building Research Establishment Ltd.: Watford, UK, 23 March 2003. [Google Scholar]
- Purser, D.A.; McAllister, J.L. Assessment of hazard to occupants from smoke, toxic gases and heat. In SFPE Handbook of Fire Protection Engineering, 5th ed.; Hurley, M.J., Gottuk, D., Hall, J.R., Harada, K., Kuligowski, E., Puchovsky, M., Torero, J., Watts, J.M., Wieczorek, C.T., Eds.; Springer: New York, NY, USA, 2016; pp. 2309–2428. [Google Scholar]
- Purser, D.A. Toxic product yield and hazard assessment for fully enclosed design fires involving fire retarded materials. Polym. Int. 2000, 49, 1232–1255. [Google Scholar] [CrossRef]
- ISO 19706:2007. Guidelines for assessing the fire threat to people.
- Blomqvist, P.; Lönnermark, A. Characterization of the combustion products in large-scale fire tests: Comparison of three experimental configurations. Fire Mater. 2001, 25, 71–81. [Google Scholar] [CrossRef]
- Blomqvist, P.; Hertzberg, T.; Tuovoinen, H. A small-scale controlled equivalence ratio tube furnace method and the link to large scale fires. In Proceedings of the 11th International Interflam Conference, London, UK, 3–5 September 2007; Interscience Communications Ltd.: London, UK, 2007; pp. 391–402. [Google Scholar]
- Andersson, B.; Markert, F.; Holmstedt, G. Combustion products generated by hetero-organic fuels on four different fire test scales. Fire Saf. J. 2005, 40, 439–465. [Google Scholar] [CrossRef]
- Babrauskas, V.; Parker, W.J.; Mulholland, G.; Twilley, W.H. The phi meter: A simple, fuel-independent instrument for monitoring combustion equivalence ratio. Rev. Sci. Instrum. 1995, 65, 2367–2375. [Google Scholar] [CrossRef]
- Purser, D.A.; Rowley, J.A.; Fardell, P.J.; Bensilum, M. Fully enclosed design fires for hazard assessment in relation to yields of carbon monoxide and hydrogen cyanide. In Proceedings of the 8th International Interflam Conference, Edinburgh, Scotland, 29 June–1 July 1999; Grayson, S., Ed.; Interscience Communications Ltd.: London, UK, 1999; Volume 2, pp. 1163–1169. [Google Scholar]
- ISO 16312-1 2010. Guidance for assessing the validity of physical fire models for obtaining fire effluent toxicity data for fire hazard and risk assessment–Part 1 Criteria.
- Purser, J.A.; Purser, D.A.; Stec, A.A.; Moffat, C.; Hull, T.R.; Su, J.Z.; Bijloos, M.; Blomqvist, P. Repeatability and reproducibility of the ISO/TS 19700 steady state tube furnace. Fire Saf. J. 2013, 55, 22–34. [Google Scholar] [CrossRef]
- Beyler, C.L. Major species production by solid fuels in a two layer compartment fire environment. In Fire Safety Science, Proceedings of the First International Symposium, August 1986; Grant, C.E., Pagni, P.P., Eds.; New York, NY, USA, 1986; pp. 431–440. [Google Scholar]
- ISO 9705:1993. Fire tests—Full-scale room test for surface products.
- Stec, A.A.; Hull, T.R.; Lebeck, K.; Purser, J.A.; Purser, D.A. The effect of temperature and ventilation condition on the toxic product yields from burning polymers. Fire Mater. 2008, 32, 49–60. [Google Scholar] [CrossRef]
- Gottuk, D.T.; Roby, R.J.; Peatross, M.; Beyler, C.L. Carbon monoxide production in compartment fires. J. Fire. Prot. Eng. 1992, 4, 133–150. [Google Scholar] [CrossRef]
- Fardell, P.J.; Purser, D.; Purser, J.; Marshall, N.; Clark, P. Fires in Reduced Oxygen Conditions. In Proceedings of the 10th International Interflam Conference, Edinburgh, UK, 5–7 July 2004; Interscience Communications Ltd.: London, UK, 2004; pp. 129–142. [Google Scholar]
- Stec, A.; Hull, T.R.; Purser, D.; Purser, J. Fire toxicity assessment: Comparison of asphyxiant yields from laboratory and large scale flaming fires. In Proceedings of the 11th International Symposium on Fire Safety Science, University of Canterbury, Christchurch, New Zealand, 10–14 February 2014.
- Alarifi, A.A.; Phylaktou, H.N.; Andrews, G.E.; Dave, J.; Aljumaiah, O.A. Toxic gas emissions from a timber-pallet-stack fire in a full-scale fire compartment. In Proceedings of the 10th Asia-Oceania Symposium on Fire Science and Technology, Tsukuba, Japan, 5–7 October 2015; Springer: Singapore, 2015. [Google Scholar]
- Pitts, W.M. An algorithm for estimating carbon monoxide formation in enclosure fires. In Fire Safety Science, Proceedings of the Fifth International Symposium, Melbourne, Australia, 3–7 March 1997; Boston, M.A., Hasemi, Y., Eds.; International Association for Fire Safety Science: Greenwich, UK, 1997; pp. 535–546. [Google Scholar]
- Purser, D.A. Fire safety performance of flame retardants compared with toxic and environmental hazards. In Polymer Green Flame Retardants; Papaspyrides, C.D., Kiliaris, P., Eds.; Elsevier: Oxford, UK, 2014; pp. 45–86. [Google Scholar]
- Lomax, S.; Simmons, R.F. The formation of carbon monoxide from diffusion flames. In Fire Safety Science, Proceedings of the First International Symposium, August 1986; Grant, C.E., Pagni, P.P., Eds.; New York, NY, USA, 1986; pp. 441–450. [Google Scholar]






| Material | Author | Compartment Fire Test Method |
|---|---|---|
| PA66 and PP a | Blomqvist and Lonnermark [15] | ISO 9705 room with variable opening in the upper part of the doorway 0.8 × 0.89, 0.68, 0.5, or 0.45 m to the exterior calorimeter hood. Fuel in 1.2 or 1.4 m2 floor pans |
| PMMA and wood b | Gottuk and Lattimer [9] | Chamber 1.57 m high × 1.22 m × 1.52 m. Air from below via 30.5-cm diameter duct and distribution plenum, variable area exhaust vent 20 cm below the ceiling. Fuel package in pan on the floor above the plenum |
| Beyler [22] | 1.0 m diameter × 0.4 m depth insulated cylindrical hood, enclosed top and sides, set at variable heights above an open burning fuel package. Effluent vented from upper 15 cm of hood into the collection plenum and duct system | |
| MDF and PIR c | Purser and Purser [7,11] | ISO 9705 room with a doorway 2 m high and width opening varied from 0.8 to 0.1 m opening to the exterior calorimeter hood. Fuel consisting of crib in floor corner configuration as the ignition source and the same fuel as the wall linings |
| Half linear scale ISO 9705 room with a doorway 1.2 m high and width varied from 0.3 to 0.05 m opening to a 4.8-m corridor. Fuel as the crib in tray on the floor in the room centre |
| Material | Method | Elemental Composition (%) | a Stoich. O2 Demand (g/g) | ||||
|---|---|---|---|---|---|---|---|
| C | H | O | N | Cl | |||
| PA66 | Analysis | 62.25 | 9.88 | 16.01 b | 11.86 | ||
| Empirical formula | 63.68 | 9.80 | 14.19 | 12.38 | 2.33 | ||
| LDPE c | Analysis | 85.50 | 14.51 | ||||
| Empirical formula | 85.63 | 14.37 | 3.42 | ||||
| Wood (P. syl) d | Analysis | 49.6 | 6.1 | 44.22 | 0.14 | 1.38 | |
| PMMA | Analysis | 60.33 | 8.14 | 31.53 a | |||
| Empirical formula | 59.98 | 8.05 | 31.96 | 1.92 | |||
| MDF | Analysis | 47.90 | 6.13 | 41.66 | 3.69 | 0.62 | 1.35 |
| PIR | Analysis e | 63.5 | 4.98 | 21.8 a | 6.15 | 3.56 | 1.87 |
| Component | Length (m) | Width (m) | Height (m) | |
|---|---|---|---|---|
| Room compartment | 1.8 | 1.2 | 1.2 | |
| Corridor | 4.8 | 0.6 | 1.2 | |
| Corridor section | upper section, effluent exit | – | 0.59 | 0.74 |
| lower section, air inlet | – | 0.59 | 0.45 | |
| Material | Rig | Expression | α | β | k1 | k2 | k3 | Φ Shift |
|---|---|---|---|---|---|---|---|---|
| PA66 | 9705 | exponential | 0.000962 | 3.7210 | 0 | |||
| PP | 9705 | exponential | 0.007729 | 2.0133 | 0 | |||
| Wood | room | Weibull | 3.2 | 18 | −0.001 | 12.6 | 4 | 0.13 |
| Wood | hood | Weibull | 3.2 | 14.65 | −0.001 | 12.6 | 7 | 0.13 |
| PMMA | room | exponential | 0.00174 | 3.4150 | 0.053 | |||
| PMMA | hood | exponential | 0.00450 | 2.8379 | 0.053 | |||
| MDF | rm-corr a | Weibull | 7 | 39 | 0 | 31 | 5.3 | Φmax |
| PIR | 9705 | linear | 0.1423 | −0.0169 | Φmax |
| Material | Average Yield (g/g) | Mean Deviation (g/g) | Mean Deviation (%) | Deviation Standard Deviation (g/g) | Range below Average (g/g) | Range above Average (g/g) | |
|---|---|---|---|---|---|---|---|
| Compartment Fire Data in Relation to Best-Fit Compartment Fire CO Curves as a Function of Φ | |||||||
| 1 | Polyamide ISO 9705 | 0.0344 | −0.0011 | −3.1 | 0.0092 | −0.0126 | 0.0224 |
| 2 | Polypropylene ISO 9705 | 0.0541 | 0.0012 | 2.2 | 0.0180 | −0.0265 | 0.0350 |
| 3 | Wood G&L a room | 0.1257 | 0.0000 | 0.0 | 0.0280 | −0.0628 | 0.0589 |
| Wood Beyler hood | 0.1125 | −0.0132 | −11.7 | 0.0276 | −0.0724 | 0.0387 | |
| 4 | PMMA G&L room | 0.1341 | 0.0167 | 13.4 | 0.1043 | −0.1872 | 0.2125 |
| 5 | MDF room-corridor | 0.1317 | −0.0016 | 1.2 | 0.0115 | −0.0102 | 0.0252 |
| MDF ISO 9705 | 0.1531 | 0.0082 | 5.3 | 0.0246 | −0.0140 | 0.0454 | |
| 6 | PIR ISO 9705 | 0.1049 | 0.0000 | 0.0 | 0.0105 | −0.0164 | 0.0148 |
| Average | 0.1063 | 0.0017 | 0.79 | 0.0292 | −0.0503 | 0.0566 | |
| 7 | Post Flashover 16312-1 | 0.24 | −0.09 | 0.09 | |||
| SSTF Data in Relation to Best-Fit Compartment Fire CO Curves as a Function of Φ | |||||||
| 1 | Polyamide v b ISO 9705 | 0.0342 | −0.0001 | −0.4 | 0.0137 | −0.0121 | 0.0296 |
| 2 | Polypropylene v ISO 9705 | 0.0556 | 0.0016 | 2.9 | 0.0078 | −0.0077 | 0.0094 |
| 3 | Wood v G&L room | 0.1250 | −0.0006 | −0.5 | 0.0214 | −0.0504 | 0.0395 |
| Wood v Beyler hood | 0.1134 | 0.0009 | 0.2 | 0.0084 | −0.0073 | 0.0145 | |
| 4 | PMMA v G&L room | 0.1343 | 0.0002 | 0.8 | 0.0146 | −0.0181 | 0.0120 |
| 5 | MDF v room-corridor | 0.1314 | −0.0003 | −0.2 | 0.0208 | −0.0330 | 0.0389 |
| 6 | PIR v ISO 9705 | 0.1052 | −0.0003 | 0.2 | 0.0200 | −0.0137 | 0.0349 |
| Average | 0.0999 | 0.0003 | 0.42 | 0.0153 | −0.0203 | 0.0267 | |
| 7 | Post Flashover 16312-1 | 0.241 | −0.091 | 0.101 c | |||
| Material | CO Yield (g/g) |
|---|---|
| Polyamide (PA6) | 0.26 |
| LDPE | 0.14 |
| MDF | 0.17 |
| MDF-fire retarded | 0.23 |
| PIR | 0.23 |
| PMMA | 0.34 |
| FPU | 0.28 |
| PAN | 0.15 |
| GRP | 0.44 |
| PVC plasticised | 0.17 |
| Average | 0.241 |
© 2016 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purser, D.A. Toxic Combustion Product Yields as a Function of Equivalence Ratio and Flame Retardants in Under-Ventilated Fires: Bench-Large-Scale Comparisons. Polymers 2016, 8, 330. https://doi.org/10.3390/polym8090330
Purser DA. Toxic Combustion Product Yields as a Function of Equivalence Ratio and Flame Retardants in Under-Ventilated Fires: Bench-Large-Scale Comparisons. Polymers. 2016; 8(9):330. https://doi.org/10.3390/polym8090330
Chicago/Turabian StylePurser, David A. 2016. "Toxic Combustion Product Yields as a Function of Equivalence Ratio and Flame Retardants in Under-Ventilated Fires: Bench-Large-Scale Comparisons" Polymers 8, no. 9: 330. https://doi.org/10.3390/polym8090330
APA StylePurser, D. A. (2016). Toxic Combustion Product Yields as a Function of Equivalence Ratio and Flame Retardants in Under-Ventilated Fires: Bench-Large-Scale Comparisons. Polymers, 8(9), 330. https://doi.org/10.3390/polym8090330