Preparation of a Novel Chitosan Based Biopolymer Dye and Application in Wood Dyeing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
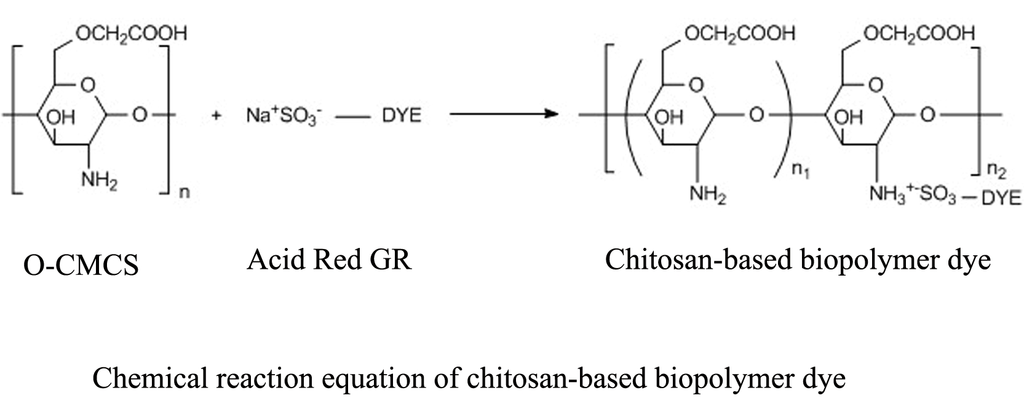
2.2. Preparation of Chitosan-Based Biopolymer Dye
2.2.1. Preparation of O-Carboxymethyl Chitosan (O-CMCS)
2.2.2. Preparation of Chitosan-Based Biopolymer Dye
2.3. Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.3.2. Degree of Substitution (DS) of O-CMCS
2.3.3. X-Ray Photoelectron Spectroscopy (XPS) Analysis
2.3.4. X-Ray Diffraction (XRD) Analysis
2.3.5. Thermogravimetric (TG) Analysis
2.3.6. Antibacterial Activity Study
2.3.7. Water Solubility Test
2.3.8. Color Difference and Color Fastness Test
2.3.9. Measurement of Dye Uptake
3. Results and Discussion
3.1. FTIR Analysis
3.2. DS of O-CMCS Analysis
3.3. XPS Analysis
3.4. XRD and Water Solubility Analysis
3.5. TG Analysis
3.6. Antibacterial Activity Study
3.7. Color Difference and Color Fastness Test
3.8. Dye Uptake Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Koev, S.T.; Dykstra, P.H.; Luo, X.; Rubloff, G.W.; Bentey, W.E.; Payne, G.F.; Ghodssi, R. Miniaturisation for chemistry, physics, biology, materials science and bioengineering. Lab Chip 2010, 10, 3026–3042. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.P.; Zhang, Z.; Gao, J.G.; Ding, C.M. Synthesis and property studies of N-carboxymethyl chitosan. J. Appl. Polym. Sci. 2011, 119, 3282–3285. [Google Scholar] [CrossRef]
- Kumar, S.; Nigam, N.; Ghosh, T.; Dutta, P.K.; Singh, S.P.; Datta, P.K.; An, L.; Shi, T.F. Preparation, characterization and optical properties of a novel azobased chitosan biopolymer. Mater. Chem. Phys. 2010, 120, 361–370. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Wolf, E.; Richter, G.; Meissner, H.; Boening, K. New concept of polymethyl methacrylate (PMMA) and polyethylene terephthalate (PET) surface coating by chitosan. Polymers 2016, 8, 132–145. [Google Scholar] [CrossRef]
- Li, X.Q.; Tang, R.C. Crosslinked and dyed chitosan fiber presenting enhanced acid resistance and bioactivities. Polymers 2016, 8, 119–132. [Google Scholar] [CrossRef]
- Sargin, I.; Kaya, M.; Arslan, G.; Baran, T.; Center, T. Preparation and characterisation of biodegradable pollen–chitosan microcapsules and its application in heavy metal removal. Bioresour. Technol. 2015, 177, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nigam, N.; Kumar, S.; Dutta, P.K.; Ghosh, T. Studies on thermo-optic property of chitosan–alizarin yellow GG complex: A direction for devices for biomedical applications. Bull. Mater. Sci. 2015, 38, 1639–1643. [Google Scholar] [CrossRef]
- Nigam, N.; Kumar, S.; Dutta, P.K.; Pei, S.; Ghosh, T. Chitosan containing azo-based Schiff bases: Thermal, antibacterial and birefringence properties for bio-optical devices. RSC Adv. 2016, 6, 5575–5581. [Google Scholar] [CrossRef]
- Nakatsuka, S.; Andrady, A.L. Permeability of vitamin B-12 in chitosan membranes. Effect of crosslinking and blending with poly(vinyl alcohol) on permeability. J. Appl. Polym. Sci. 1992, 44, 17–28. [Google Scholar] [CrossRef]
- Busilacchi, A.; Gigante, A.; Mattioli-Belmonte, M.; Manzotti, S.; Muzzarelli, R.A. Chitosan stabilizes platelet growth factors and modulates stem cell differentiation toward tissue regeneration. Carbohydr. Polym. 2013, 98, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, J.F.; Kan, J.; Tang, Y.Q.; Jin, C.H. Preparation, characterization and antioxidant activity of phenolic acids grafted carboxymethyl chitosan. Int. J. Biol. Macromol. 2013, 62, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Q.; Lin, D.; Yao, S. Effect and mechanism of sodium chloride on the formation of chitosan–cellulose sulfate–tripolyphosphate crosslinked beads. Soft Matter 2013, 9, 10354–10363. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.M.; Ravindra, K.B. Bioactive antimicrobial agents for finishing of textiles for health care products. J. Text. Inst. 2015, 106, 706–717. [Google Scholar] [CrossRef]
- Kumar, S.; Nigam, N.; Ghosh, T.; Dutta, P.K.; Singh, S.P.; Datta, P.K. Studies on chitosan-alizarin yellow GG complex for optical and biomedical applications. J. Polym. Mater. 2009, 26, 411–416. [Google Scholar]
- Wang, J.P.; Chen, Y.Z.; Wang, Y.; Yuan, S.J.; Sheng, G.P.; Yu, H.Q. A novel efficient cationic flocculant prepared through grafting two monomers ontochitosan induced by Gamma radiation. RSC Adv. 2012, 2, 494–500. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Xing, R.E.; Liu, S.; Zhong, Z.M.; Ji, X.; Wang, L.; Li, P.C. Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydr. Res. 2007, 342, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, V.E.; Stepnova, E.A.; Babak, V.G.; Krayukhina, M.A.; Berezin, B.B.; Yamskov, I.A. Amphiphilic N-[2(3)-(dodec-2’-en-1’-yl)succinoyl] chitosan: Synthesis and properties. React. Funct. Polym. 2008, 68, 436–445. [Google Scholar] [CrossRef]
- Sashiwa, H.; Yamamori, N.; Ichinose, Y.; Sunamoto, J.; Aiba, S.I. Chemical modification of chitosan. Macromol. Biosci. 2003, 3, 231–233. [Google Scholar] [CrossRef]
- Garg, P.; Kumar, S.; Pandey, S.; Seonwoo, H.; Choung, P.H.; Koh, J.; Chung, J.H. Triphenylamine coupled chitosan with high buffering capacity and low viscosity for enhanced transfection in mammalian cells, in vitro and in vivo. J. Mater. Chem. B 2013, 1, 6053–6065. [Google Scholar] [CrossRef]
- Chen, H.; Chen, M.; Wang, X.; Sun, R.S. Self-assembled conjugated polymer/carboxymethyl chitosan grafted poly(p-dioxanone) nanomicelles and their use in functionalized indicator paper for fast and visual detection of a banned food dye. Polym. Chem. 2014, 5, 4251–4258. [Google Scholar] [CrossRef]
- Tang, R.; Yu, Z.; Zhang, Y. Synthesis, characterization, and properties of antibacterial dye based on chitosan. Cellulose 2016, 23, 1741–1749. [Google Scholar] [CrossRef]
- Lee, Y.H.; Hwang, E.K.; Baek, Y.M.; Kim, H.D. Deodorizing function and antibacterial activity of fabrics dyed with gallnut (Galla chinensis) extract. Text. Res. J. 2015, 85, 1045–1054. [Google Scholar] [CrossRef]
- Romagnoli, M.; Segoloni, E.; Luna, M.; Margaritelli, A.; Gatti, M.; Santamaria, U.; Vinciguerra, V. Wood color in Lapacho (Tabebuia serratifolia): Chemical composition and industrial implications. Wood Sci. Technol. 2013, 47, 701–716. [Google Scholar] [CrossRef]
- Tang, R.L.; Zhang, Y.; Zhang, Y.; Yu, Z.M. Synthesis and characterization of chitosan based dye containing quaternary ammonium group. Carbohydr. Polym. 2016, 139, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Pandey, A.C.; Verma, S.P.; Das, P.; Tewari, R.P. Efficient water soluble nanostructured ZnO grafted O-carboxymethyl chitosan/curcumin-nanocomposite for cancer therapy. Process Biochem. 2015, 50, 678–688. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Sarkar, K.; Soam, S.; Kundu, P.P. Formulation of pH-responsive carboxymethyl chitosan and alginate beads for the oral delivery of insulin. J. Appl. Polym. Sci. 2013, 129, 835–845. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Murray, J.W. The surface chemistry of goethite (α-FeOOH) in major ion seawater. Am. J. Sci. 1981, 281, 788–806. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Lazaridis, N.K. Reactive and basic dyes removal by sorption onto chitosan derivatives. J. Colloid Interface Sci. 2009, 331, 32–39. [Google Scholar]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 2013, 91, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, H.; She, Y.; Pu, J.W. Composite films of N,O-carboxymethyl chitosan and bamboo fiber. J. Appl. Polym. Sci. 2014, 131, 39851–39856. [Google Scholar] [CrossRef]
- Chi, W.; Li, W.; Jin, Y.; Zhang, R.; Peng, G.; Qin, C. Synthesis of 2-hydroxypropyltrimethyl ammonium chloride chitosan. J. XiaoGan Univ. 2006, 6, 19–21,39. (In Chinese) [Google Scholar]
- Lee, Y.H.; Kim, H.D. Dyeing properties and colour fastness of cotton and silk fabrics dyed with Cassia tora L. extract. Fibers Polym. 2004, 5, 303–308. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.H.; Gao, J.M.; Guo, H.W.; Chen, Y.; Cheng, Q.Z.; Via, B.K. Wood veneer dyeing enhancement by ultrasonic-assisted treatment. Bioresources 2015, 10, 1198–1212. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argulles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Zheng, M.; Han, B.; Yang, Y.; Liu, W. Synthesis, characterization and biological safety of O-carboxymethyl chitosan used to treat Sarcoma 180 tumor. Carbohydr. Polym. 2011, 86, 231–238. [Google Scholar] [CrossRef]
- Lv, J.; Zhou, Q.; Liu, G.; Gao, D.; Wang, C. Preparation and properties of polyester fabrics grafted with O-carboxymethyl chitosan. Carbohydr. Polym. 2014, 113, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Du, Y.; Fan, L.; Chen, X.; Yang, J. Preparation, characterization and antimicrobial activity of quaternized carboxymethyl chitosan and application as pulp-cap. Polymer 2006, 47, 1796–1804. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K.D. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Tang, R.L.; Yu, Z.M.; Zhang, Y.; Qi, C.S. Mechanisms and properties of chitosan-assisted bamboo dyeing. BioResoures 2015, 10, 3326–3336. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Wang, A.Q. Adsorption of cationic dye on N,O-carboxymethyl-chitosan from aqueous solutions: Equilibrium, kinetics, and adsorption mechanism. Polym. Bull. 2010, 65, 961–975. [Google Scholar] [CrossRef]
- Spinelli, V.A.; Laranjeira, M.C.M.; Fávere, V.T. Preparation and characterization of quaternary chitosan salt: Adsorption equilibrium of chromium(VI) ion. React. Funct. Polym. 2004, 61, 347–352. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Z.M.; Zhang, Y.; Tang, R.L. Color fixation mechanism of CTS-Ag and its effect on the properties of dyed veneer. J. Beijing For. Univ. 2015, 37, 107–111. (In Chinese) [Google Scholar]
- Song, X.Y.; Shen, Y.R. Dyeing with the Reactive Dyes; China Textile Press: Beijing, China, 2009; pp. 550–558. [Google Scholar]







| Sample | Tei (°C) | Tmax1 (°C) | Tmax2 (°C) | Char Residue at 700 °C (%) |
|---|---|---|---|---|
| Chitosan | 85 | 88 | 291 | 33.5 |
| O-CMCS | 49 | 95 | 221 | 30.5 |
| Chitosan-based biopolymer dye | 46 | 101 | 233 | 34.1 |
| Sample | S. aureus | E. coil | ||||
|---|---|---|---|---|---|---|
| Concentration of Bacteria (CFU/cm2) | Reduction (%) | Concentration of Bacteria (CFU/cm2) | Reduction (%) | |||
| 0 h | 24 h | 0 h | 24 h | |||
| Blank control | 1.8 × 104 | 1.8 × 106 | – | 2.3 × 104 | 1.7 × 106 | – |
| Dyed poplar wood with Acid Red GR | 1.7 × 104 | 4.2 × 103 | 72 | 2.2 × 104 | 4.1 × 103 | 78 |
| Dyed poplar wood with chitosan based biopolymer dye | – | <1.3 | >99 | – | <1.3 | >99 |
| Color Parameters | Dye | Chitosan-Based Biopolymer Dye | Magnitude 1 |
|---|---|---|---|
| L* | 70.98 ± 1.87 | 68.96 ± 1.17 | −2.03 ± 2.04 |
| a* | 19.37 ± 2.19 | 22.73 ± 2.01 | 3.36 ± 1.95 |
| b* | 19.50 ± 1.26 | 20.35 ± 0.81 | 0.86 ± 0.99 |
| ΔE* 2 | – | 4.82 ± 0.62 | – |
| Color Parameter 1 | Water-Fastness | Light-Fastness | ||
|---|---|---|---|---|
| Dye | Chitosan-Based Biopolymer Dye | Dye | Chitosan-Based Biopolymer Dye | |
| ΔL* | 5.36 ± 0.34 | 2.10 ± 0.79 | −8.45 ± 1.17 | −1.98 ± 0.40 |
| Δa* | −12.53 ± 0.35 | −8.38 ± 0.19 | −10.20 ± 0.40 | −3.62 ± 0.09 |
| Δb* | −3.03 ± 0.24 | −5.44 ± 0.45 | −0.14 ± 1.08 | 6.15 ± 0.74 |
| ΔE* 2 | 13.96 ± 0.40 | 10.23 ± 0.42 | 13.29 ± 0.99 | 7.43 ± 0.63 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tang, R.; Zhang, Y.; Yu, Z.; Qi, C. Preparation of a Novel Chitosan Based Biopolymer Dye and Application in Wood Dyeing. Polymers 2016, 8, 338. https://doi.org/10.3390/polym8090338
Wang X, Tang R, Zhang Y, Yu Z, Qi C. Preparation of a Novel Chitosan Based Biopolymer Dye and Application in Wood Dyeing. Polymers. 2016; 8(9):338. https://doi.org/10.3390/polym8090338
Chicago/Turabian StyleWang, Xiaoqian, Ruilin Tang, Yang Zhang, Zhiming Yu, and Chusheng Qi. 2016. "Preparation of a Novel Chitosan Based Biopolymer Dye and Application in Wood Dyeing" Polymers 8, no. 9: 338. https://doi.org/10.3390/polym8090338
APA StyleWang, X., Tang, R., Zhang, Y., Yu, Z., & Qi, C. (2016). Preparation of a Novel Chitosan Based Biopolymer Dye and Application in Wood Dyeing. Polymers, 8(9), 338. https://doi.org/10.3390/polym8090338