Modification of Thermal and Mechanical Properties of PEG-PPG-PEG Copolymer (F127) with MA-POSS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of F127-POSS Copolymers by Atom Transfer Radical Polymerization (ATRP)
2.2. Molecular Characterisation
2.3. Thermal Degradation Characterisation
2.4. Rheological Measurements
3. Results
3.1. Synthesis of Polymers
3.2. Thermal Degradation Analysis
3.3. Thermal Gelling Behaviour
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naik, A.; Kalia, Y.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar] [PubMed]
- Kurumada, K.-I.; Robinson, B.H. Viscosity studies of pluronic F127 in aqueous solution. In Trends in Colloid and Interface Science XVI; Miguel, M., Burrows, D.H., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2004. [Google Scholar]
- Loh, C.H.; Wang, R. Effects of additives and coagulant temperature on fabrication of high performance PVDF/Pluronic F127 blend hollow fiber membranes via nonsolvent induced phase separation. Chin. J. Chem. Eng. 2012, 20, 71–79. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, S.J.; Kim, D.; Lee, D.S.; Kim, S.C. Micelle formation and sol-gel transition behavior of comb-like amphiphilic poly((PLGA-b-PEG)MA) copolymers. J. Polym. Sci. Part A 2008, 46, 1954–1963. [Google Scholar] [CrossRef]
- Tsao, C.T.; Hsiao, M.H.; Zhang, M.; Levengood, S.L.; Zhang, M. Chitosan-PEG hydrogel with sol-gel transition triggerable by multiple external stimuli. Macromol. Rapid Commun. 2015, 36, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Kadla, J.F.; Korehei, R. Effect of hydrophilic and hydrophobic interactions on the rheological behavior and microstructure of a ternary cellulose acetate system. Biomacromolecules 2010, 11, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-W.; Chang, F.-C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Loh, X.J.; Zhang, Z.X.; Mya, K.Y.; Wu, Y.L.; He, C.B.; Li, J. Efficient gene delivery with paclitaxel-loaded DNA-hybrid polyplexes based on cationic polyhedral oligomeric silsesquioxanes. J. Mater. Chem. 2010, 20, 10634–10642. [Google Scholar] [CrossRef]
- Wu, J.; Mather, P.T. POSS polymers: Physical properties and biomaterials applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Cai, X.; Yuan, J.; Chen, S.; Li, P.; Li, L.; Shen, J. Hemocompatibility improvement of poly(ethylene terephthalate) via self-polymerization of dopamine and covalent graft of zwitterions. Mater. Sci. Eng. 2014, 36, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Hussain, H.; He, C.B. Tailoring micelle formation and gelation in (PEG-p(MA-POSS)) amphiphilic hybrid block copolymers. Macromolecules 2011, 44, 622–631. [Google Scholar] [CrossRef]
- Mya, K.Y.; Gose, H.B.; Pretsch, T.; Bothe, M.; He, C. Star-shaped POSS-polycaprolactone polyurethanes and their shape memory performance. J. Mater. Chem. 2011, 21, 4827–4836. [Google Scholar] [CrossRef]
- Bothe, M.; Mya, K.Y.; Lin, E.M.J.; Yeo, C.C.; Lu, X.; He, C.; Pretsch, T. Triple-shape properties of star-shaped POSS-polycaprolactone polyurethane networks. Soft Matter 2012, 8, 965–972. [Google Scholar] [CrossRef]
- Tan, B.H.; Hussain, H.; Leong, Y.W.; Lin, T.T.; Tjiu, W.W.; He, C. Tuning self-assembly of hybrid PLA-p(MA-POSS) block copolymers in solution via stereocomplexation. Polym. Chem. 2013, 4, 1250–1259. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, B.H.; Hussain, H.; He, C. PH-responsive amphiphilic hybrid random-type copolymers of poly(acrylic acid) and poly(acrylate-poss): Synthesis by atrp and self-assembly in aqueous solution. Colloid Polym. Sci. 2013, 291, 1803–1815. [Google Scholar] [CrossRef]
- Lewicki, J.P.; Pielichowski, K.; De La Croix, P.T.; Janowski, B.; Todd, D.; Liggat, J.J. Thermal degradation studies of polyurethane/POSS nanohybrid elastomers. Polym. Degrad. Stab. 2010, 95, 1099–1105. [Google Scholar] [CrossRef]
- Tokunaga, T.; Koge, S.; Mizumo, T.; Ohshita, J.; Kaneko, Y. Facile preparation of a soluble polymer containing polyhedral oligomeric silsesquioxane units in its main chain. Polym. Chem. 2015, 6, 3039–3045. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Cozzo, G.; Latteri, A.; Recca, A. Synthesis and thermal characterization of new dumbbell shaped POSS/PS nanocomposites: Influence of the symmetrical structure of the nanoparticles on the dispersion/aggregation in the polymer matrix. Polym. Compos. 2015, 36, 1394–1400. [Google Scholar] [CrossRef]
- Chinnam, P.R.; Zhang, H.; Wunder, S.L. Blends of pegylated polyoctahedralsilsesquioxanes (POSS-PEG) and methyl cellulose as solid polymer electrolytes for lithium batteries. Electrochim. Acta 2015, 170, 191–201. [Google Scholar] [CrossRef]
- Pyun, J.; Matyjaszewski, K.; Wu, J.; Kim, G.-M.; Chun, S.B.; Mather, P.T. ABA triblock copolymers containing polyhedral oligomeric silsesquioxane pendant groups: Synthesis and unique properties. Polymer 2003, 44, 2739–2750. [Google Scholar] [CrossRef]
- Wang, W.; Sun, X.; Huang, L.; Gao, Y.; Ban, J.; Shen, L.; Chen, J. Structure–property relationships in hybrid dental nanocomposite resins containing monofunctional and multifunctional polyhedral oligomeric silsesquioxanes. Int. J. Nanomed. 2014, 9, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Nam, J.G.; Wilhellm, M.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear behavior of PEO-PPO-PEO triblock copolymer solutions. Rheol. Acta 2005, 45, 239–249. [Google Scholar] [CrossRef]
- Loh, X.J.; Zhang, Z.-X.; Wu, Y.-L.; Lee, T.S.; Li, J. Synthesis of novel biodegradable thermoresponsive triblock copolymers based on poly((R)-3-hydroxybutyrate) and poly(N-isopropylacrylamide) and their formation of thermoresponsive micelles. Macromolecules 2008, 42, 194–202. [Google Scholar] [CrossRef]
- Loh, X.J.; Tan, Y.X.; Li, Z.; Teo, L.S.; Goh, S.H.; Li, J. Biodegradable thermogelling poly(ester urethane)s consisting of poly(lactic acid)—Thermodynamics of micellization and hydrolytic degradation. Biomaterials 2008, 29, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J.; Ong, S.J.; Tung, Y.T.; Choo, H.T. Incorporation of poly((R)-3-hydroxybutyrate) into cationic copolymers based on poly(2-(dimethylamino)ethyl methacrylate) to improve gene delivery. Macromol. Biosci. 2013, 13, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Tan, B.H.; Seah, G.L.; Liu, Y.; He, C.B.; Davis, T.P. Micelle formation and gelation of (PEG-p(MA-POSS)) amphiphilic block copolymers via associative hydrophobic effects. Langmuir 2010, 26, 11763–11773. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tan, B.H.; Jin, G.; Li, K.; He, C. Design of polyhedral oligomeric silsesquioxane (POSS) based thermo-responsive amphiphilic hybrid copolymers for thermally denatured protein protection applications. Polym. Chem. 2014, 5, 6740–6753. [Google Scholar] [CrossRef]
- Dou, Q.Q.; Liow, S.S.; Ye, E.; Lakshminarayanan, R.; Loh, X.J. Biodegradable thermogelling polymers: Working towards clinical applications. Adv. Healthc. Mater. 2014, 3, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Wang, H.; Qiu, Y.-K.; Loh, X.J. PLA-based thermogel for the sustained delivery of chemotherapeutics in a mouse model of hepatocellular carcinoma. RSC Adv. 2016, 6, 44506–44513. [Google Scholar] [CrossRef]
- Liow, S.S.; Karim, A.A.; Loh, X.J. Biodegradable thermogelling polymers for biomedical applications. MRS Bull. 2016, 41, 557–566. [Google Scholar] [CrossRef]
- Liow, S.S.; Dou, Q.; Kai, D.; Karim, A.A.; Zhang, K.; Xu, F.; Loh, X.J. Thermogels: In situ gelling biomaterial. ACS Biomater. Sci. Eng. 2016, 2, 295–316. [Google Scholar] [CrossRef]
- Jiang, L.; Gan, C.R.R.; Gao, J.; Loh, X.J. A perspective on the trends and challenges facing porphyrin-based anti-microbial materials. Small 2016. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Loh, X.J. Polyhydroxyalkanoates: Chemical modifications toward biomedical applications. ACS Sustain. Chem. Eng. 2014, 2, 106–119. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Tan, M.J.; Thoniyot, P.; Loh, X.J. Unusual thermogelling behaviour of poly(2-(dimethylamino)ethyl methacrylate) (pdmaema)-based polymers polymerized in bulk. RSC Adv. 2015, 5, 62314–62318. [Google Scholar] [CrossRef]
- Loh, X.J.; Gan, H.X.; Wang, H.; Tan, S.J.E.; Neoh, K.Y.; Tan, S.S.J.; Diong, H.F.; Kim, J.J.; Lee, W.L.S.; Fang, X.T.; et al. New thermogelling poly(ether carbonate urethane)s based on Pluronics F127 and poly(polytetrahydrofuran carbonate). J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- Loh, X.J. Supramolecular host-guest polymeric materials for biomedical applications. Mater. Horiz. 2014, 1, 185–195. [Google Scholar] [CrossRef]
- Loh, X.J.; Goh, S.H.; Li, J. Hydrolytic degradation and protein release studies of thermogelling polyurethane copolymers consisting of poly((R)-3-hydroxybutyrate), poly(ethylene glycol), and poly(propylene glycol). Biomaterials 2007, 28, 4113–4123. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.N.; Kuo, N.Y.; Loh, X.J. New biocompatible thermogelling copolymers containing ethylene-butylene segments exhibiting very low gelation concentrations. Soft Matter 2011, 7, 2150–2159. [Google Scholar] [CrossRef]
- Loh, X.J.; Vu, P.N.N.; Kuo, N.Y.; Li, J. Encapsulation of basic fibroblast growth factor in thermogelling copolymers preserves its bioactivity. J. Mater. Chem. 2011, 21, 2246–2254. [Google Scholar] [CrossRef]
- Loh, X.J.; Cheng, L.W.I.; Li, J. Micellization and thermogelation of poly(ether urethane)s comprising poly(ethylene glycol) and poly(propylene glycol). In Modern Trends in Polymer Science-EPF’09; Stelzer, F., Wiesbrock, E., Eds.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Sugimoto, M.; Suzuki, Y.; Hyun, K.; Ahn, K.H.; Ushioda, T.; Nishioka, A.; Taniguchi, T.; Koyama, K. Melt rheology of long-chain-branched polypropylenes. Rheol. Acta 2005, 46, 33–44. [Google Scholar] [CrossRef]
- Hyun, K.; Nam, J.G.; Wilhelm, M.; Ahn, K.H.; Lee, S.J. Nonlinear response of complex fluids under laos (large amplitude oscillatory shear) flow. Korea Aust. Rheol. J. 2003, 15, 97–105. [Google Scholar]
- Dealy, J.M.; Wissbrun, K.F. Melt Rheology and Its Role in Plastics Processing: Theory and Applications; Springer: Berlin, Germany, 2012. [Google Scholar]
- Rogers, S.; Kohlbrecher, J.; Lettinga, M.P. The molecular origin of stress generation in worm-like micelles, using a rheo-SANS LAOS approach. Soft Matter 2012, 8, 7831–7839. [Google Scholar] [CrossRef]
- McKinley, G.H.; Ewoldt, R.H.; Ng, T.; Dimitriou, C. Rheological Fingerprinting: Using LAOS to Physically Interpret the Nonlinear Behavior of Complex Fluids and Soft Solids. Available online: http://www.rheology.org.au/Resources/LectureSeries/ASR-Lecture-2012-January_McKinley_slideshow.pdf (accessed on 3 May 2016).
- Garboczi, E.J.; Bentz, D.P.; Snyder, K.A.; Martys, N.S.; Stutzman, P.E.; Ferraris, C.F.; Bullard, J.W. An Electronic Monograph: Modeling and Measuring the Structure and Properties of Cement-Based Materials. Available online: http://ciks.cbt.nist.gov/~garbocz/SP946/node11.htm (accessed on 9 September 2016).
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A review of nonlinear oscillatory shear tests: Analysis and application of large amplitude oscillatory shear (LAOS). Prog. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]
- Ewoldt, R.H.; Winter, P.; Maxey, J.; McKinley, G.H. Large amplitude oscillatory shear of pseudoplastic and elastoviscoplastic materials. Rheol. Acta 2010, 49, 191–212. [Google Scholar] [CrossRef]
- Majumdar, P.; He, J.; Lee, E.; Kallam, A.; Gubbins, N.; Stafslien, S.J.; Daniels, J.; Chisholm, B.J. Antimicrobial activity of polysiloxane coatings containing quaternary ammonium-functionalized polyhedral oligomeric silsesquioxane. J. Coat. Technol. Res. 2010, 7, 455–467. [Google Scholar] [CrossRef]







| Sample | Initiator Br-F127-Br | Monomer MA-POSS | Ligand HMTETA | Catalyst CuBr |
|---|---|---|---|---|
| F127-4POSS | 1.25 g | 0.3909 g | 0.107 mL | 28 mg |
| F127-8POSS | 1.25 g | 0.7819 g | 0.107 mL | 28 mg |
| Samples | Onset, 5 wt % loss (°C) | 10 wt % loss (°C) | Max degradation rate (°C) | Weight remaining |
|---|---|---|---|---|
| F127-POSS | 339.6 | 366.0 | 417.6 | 1.2 wt % |
| F127 | 305.6 | 337.7 | 403.9 | 0.6 wt % |
| Tests performed | Polymer composition | Sinusoidal waveform/Lissajous curves | Inference | |
|---|---|---|---|---|
| F127 (Control) | F127 (Control) | |||
| Oscillation temperature sweep | Gelation Moduli range: 103 Pa TGelation: 23.5 °C | Gelation Moduli range: 103 Pa TGelation: 33.5 °C | In the presence of POSS, the gelation temperature was elevated, without compromising the gel strength (modulus range) | |
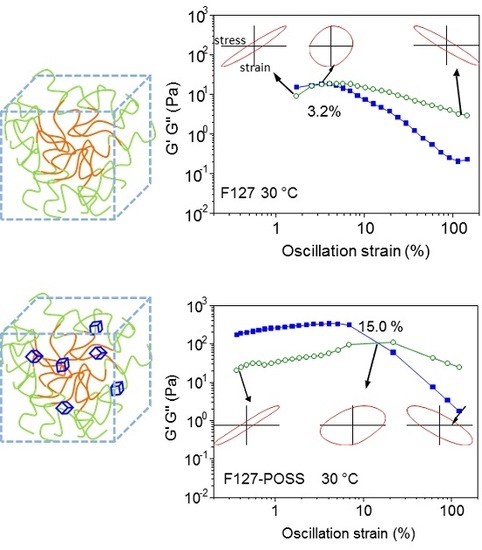
| Oscillation amplitude sweep | At 25 °C Moduli range: 1 Pa; No G’-G” crossover point | At 25 °C Moduli range: 1 Pa; No G’-G” crossover point | Narrow elliptical form, displacement waveform 180° out of phase with torque throughout entire oscillatory amplitude sweep, indicating liquid form | Modulus increased when temperature increased from 25 to 30 °C—indicative of the temperature-responsive property not altered for POSS-reinforced polymer. The G’-G” crossover point increased from 3.2% (F127) to 15.0% (F127-POSS), suggested F127-POSS matrix dissipated more energy as compared to F127 before the collapse of the microstructure for the same amount of stress applied |
| At 30 °C Moduli range: 10−1–101 Pa Crossover G’-G”: 3.2% strain | At 30 °C Moduli range: 100–102 Pa Crossover G’-G”: 15% strain | F127-POSS larger curve area compared to F127 (most prominent in endpoint Lissajous curves), indicating F127-POSS dissipated more energy than F127 | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, Q.; Abdul Karim, A.; Loh, X.J. Modification of Thermal and Mechanical Properties of PEG-PPG-PEG Copolymer (F127) with MA-POSS. Polymers 2016, 8, 341. https://doi.org/10.3390/polym8090341
Dou Q, Abdul Karim A, Loh XJ. Modification of Thermal and Mechanical Properties of PEG-PPG-PEG Copolymer (F127) with MA-POSS. Polymers. 2016; 8(9):341. https://doi.org/10.3390/polym8090341
Chicago/Turabian StyleDou, Qingqing, Anis Abdul Karim, and Xian Jun Loh. 2016. "Modification of Thermal and Mechanical Properties of PEG-PPG-PEG Copolymer (F127) with MA-POSS" Polymers 8, no. 9: 341. https://doi.org/10.3390/polym8090341
APA StyleDou, Q., Abdul Karim, A., & Loh, X. J. (2016). Modification of Thermal and Mechanical Properties of PEG-PPG-PEG Copolymer (F127) with MA-POSS. Polymers, 8(9), 341. https://doi.org/10.3390/polym8090341