Supported Ionic Liquid Silica as Curing Agent for Epoxy Composites with Improved Mechanical and Thermal Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Supported Ionic Liquid Silica (IL-Silica)
2.3. Composite Fabrication
2.4. Characterizations
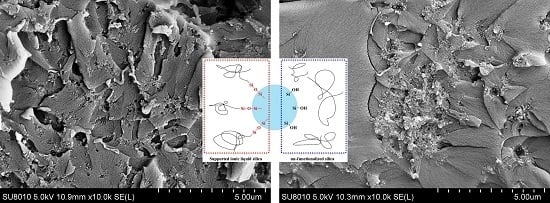
3. Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chrusciel, J.J.; Lesniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Meer, S.; Kausar, A.; Iqbal, T. Attributes of polymer and silica nanoparticle composites: A review. Polym. Plast. Technol. Eng. 2016, 55, 826–861. [Google Scholar] [CrossRef]
- Sandomierski, M.; Strzemiecka, B.; Chehimi, M.M.; Voelkel, A. Reactive diazonium-modified silica fillers for high-performance polymers. Langmuir 2016, 32, 11646–11654. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Chao, M.Y.; Hui, D.; Liu, J.; Zheng, J.C.; Lei, W.W.; Zhou, X.X.; Wang, R.G.; Zhang, L.Q. Enhanced interfacial interaction and excellent performance of silica/epoxy group-functionalized styrene-butadiene rubber (SBR) nanocomposites without any coupling agent. Compos. Part B Eng. 2017, 114, 356–364. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, S.Q.; Peng, J.; Liu, L. The filler-rubber interface and reinforcement in styrene butadiene rubber composites with graphene/silica hybrids: A quantitative correlation with the constrained region. Compos. Part A Appl. Sci. Manuf. 2016, 86, 19–30. [Google Scholar] [CrossRef]
- Legrand, A.; Soulie-Ziakovic, C. Silica-epoxy vitrimer nanocomposites. Macromolecules 2017, 109, 339–348. [Google Scholar] [CrossRef]
- Guenthner, A.J.; Sahagun, C.M.; Lamison, K.R.; Reams, J.T.; Haddad, T.S.; Mabry, J.M. Effect of nanoparticle functionalization on the performance of polycyanurate/silica nanocomposites. Ind. Eng. Chem. Res. 2016, 55, 7096–7107. [Google Scholar] [CrossRef]
- Tang, Z.H.; Huang, J.; Wu, X.H.; Guo, B.C.; Zhang, L.Q.; Liu, F. Interface engineering toward promoting silanization by ionic liquid for high-performance rubber/silica composites. Ind. Eng. Chem. Res. 2015, 54, 10747–10756. [Google Scholar] [CrossRef]
- Xu, T.W.; Jia, Z.X.; Luo, Y.F.; Jia, D.M.; Peng, Z. Interfacial interaction between the epoxidized natural rubber and silica in natural rubber/silica composites. Appl. Surf. Sci. 2015, 328, 306–313. [Google Scholar] [CrossRef]
- Badwe, N.; Mahajan, R.; Sieradzki, K. Interfacial fracture strength and toughness of copper/epoxy-resin interfaces. Acta Mater. 2016, 103, 512–518. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.Q.; Xu, C.X.; Liu, Y.; Dai, J.Y.; Wang, Z.B.; Liu, X.Q.; Chen, J.; Shen, X.B.; Wei, J.J. Vanillin-derived high-performance flame retardant epoxy resins: Facile synthesis and properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Rohde, B.J.; Le, K.M.; Krishnamoorti, R.; Robertson, M.L. Thermoset Blends of an epoxy resin and polydicyclopentadiene. Macromolecules 2016, 49, 8960–8970. [Google Scholar] [CrossRef]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef]
- Heng, Z.G.; Chen, Y.; Zou, H.W.; Liang, M. Simultaneously enhanced tensile strength and fracture toughness of epoxy resins by a poly(ethylene oxide)-block-carboxyl terminated butadiene-acrylonitrile rubber dilock copolymer. RSC Adv. 2015, 5, 42362–42368. [Google Scholar] [CrossRef]
- Mansour, G.; Tsongas, K.; Tzetzis, D. Investigation of the dynamic mechanical properties of epoxy resins modified with elastomers. Compos. Part B Eng. 2016, 94, 152–159. [Google Scholar]
- Lin, J.J.; Bang, S.H.; Malakooti, M.H.; Sodano, H.A. Isolation of aramid nanofibers for high strength and toughness polymer nanocomposites. ACS. Appl. Mater. Interfaces 2017, 9, 11167–11175. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.B.; Ma, C.; Gu, J.W.; Guo, J.; Yan, X.R.; Huang, J.N.; Zhang, Q.Y.; Guo, Z.H. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Domun, N.; Hadavinia, H.; Zhang, T.; Sainsbury, T.; Liaghat, G.H.; Vahid, S. Improving the fracture toughness and the strength of epoxy using nanomaterials—A review of the current status. Nanoscale 2015, 7, 10294–10329. [Google Scholar] [CrossRef] [PubMed]
- Marouf, B.T.; Mai, Y.W.; Bagheri, R.; Pearson, R.A. Toughening of epoxy nanocomposites: Nano and hybrid effects. Polym. Rev. 2016, 56, 70–112. [Google Scholar] [CrossRef]
- Kothmann, M.H.; Zeiler, R.; de Anda, A.R.; Bruckner, A.; Altstadt, V. Fatigue crack propagation behaviour of epoxy resins modified with silica-nanoparticles. Polymer 2015, 60, 157–163. [Google Scholar] [CrossRef]
- Kang, T.; Lee, J.H.; Oh, S.G. Dispersion of surface-modified silica nanoparticles in polyamide-imide (PAI) films for enhanced mechanical and thermal properties. J. Ind. Eng. Chem. 2017, 46, 289–297. [Google Scholar] [CrossRef]
- Peng, Z.C.; Li, Q.; Li, H.Y.; Hu, Y.L. Polyethylene-modified nano silica and its fine dispersion in polyethylene. Ind. Eng. Chem. Res. 2017, 56, 5892–5898. [Google Scholar] [CrossRef]
- Kang, D.J.; Park, G.U.; Park, H.Y.; Im, H.G. A robust transparent encapsulation material: Silica nanoparticle-embedded epoxy hybrid nanocomposite. Compos. Sci. Technol. 2017, 144, 107–113. [Google Scholar] [CrossRef]
- Chen, L.J.; Jia, Z.X.; Tang, Y.H.; Wu, L.H.; Luo, Y.F.; Jia, D.M. Novel functional silica nanoparticles for rubber vulcanization and reinforcement. Compos. Sci. Technol. 2017, 144, 11–17. [Google Scholar] [CrossRef]
- Rahmathullah, M.A.M.; Jeyarajasingam, A.; Merritt, B.; VanLandingham, M.; McKnight, S.H.; Palmese, G.R. Room Temperature ionic liquids as thermally latent initiators for polymerization of epoxy resins. Macromolecules 2009, 42, 3219–3221. [Google Scholar] [CrossRef]
- Maka, H.; Spychaj, T. Epoxy resin crosslinked with conventional and deep eutectic ionic liquids. Polimery 2012, 6, 456–462. [Google Scholar] [CrossRef]
- Maka, H.; Spychaj, T.; Pilawka, R. Epoxy resin/phosphonium ionic liquid/carbon nanofiller systems: Chemorheology and properties. Express Polym. Lett. 2014, 8, 723–732. [Google Scholar] [CrossRef] [Green Version]
- Maka, H.; Spychaj, T.; Pilawka, R. Epoxy resin/ionic liquid systems: The influence of imidazolium cation size and anion type on reactivity and thermomechanical properties. Ind. Eng. Chem. Res. 2012, 51, 5197–5206. [Google Scholar] [CrossRef]
- Li, S.P.; Cui, C. Enhancing the mechanical properties of epoxy resin by addition of an amino-terminated hyperbranched polymer grown on glass fiber. J. Mater. Sci. 2016, 51, 1829–1837. [Google Scholar] [CrossRef]
- Zhang, L.X.; Jiao, H.Q.; Jiu, H.F.; Chang, J.X.; Zhang, S.M.; Zhao, Y.A. Thermal, mechanical and electrical properties of polyurethane/(3-aminopropyl) triethoxysilane functionalized graphene/epoxy resin interpenetrating shape memory polymer composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 286–295. [Google Scholar] [CrossRef]
- Pour, Z.S.; Ghaemy, M. Polymer grafted graphene oxide: For improved dispersion in epoxy resin and enhancement of mechanical properties of nanocomposite. Compos. Sci. Technol. 2016, 136, 145–157. [Google Scholar] [CrossRef]
- Wu, Y.P.; Chen, M.B.; Chen, M.; Ran, Z.P.; Zhu, C.H.; Liao, H. The reinforcing effect of polydopamine functionalized graphene nanoplatelets on the mechanical properties of epoxy resins at cryogenic temperature. Polym. Test. 2017, 58, 262–269. [Google Scholar] [CrossRef]
- Jeyranpour, F.; Alahyarizadeh, G.; Minuchehr, A. The thermo-mechanical properties estimation of fullerene-reinforced resin epoxy composites by molecular dynamics simulation—A comparative study. Polymer 2016, 88, 9–18. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sellaiyan, S.; Kakizaki, T.; Uedono, A.; Taniguchi, Y.; Hayashi, K. Effect of free-volume holes on dynamic mechanical properties of epoxy resins for carbon-fiber-reinforced polymers. Macromolecules 2017, 50, 3934–3943. [Google Scholar] [CrossRef]
- Gu, J.W.; Yang, X.T.; Lv, Z.Y.; Li, N.; Liang, C.B.; Zhang, Q.Y. Functionalized graphite nanoplatelets/epoxy resin nanocomposites with high thermal conductivity. Int. J. Heat Mass Transf. 2016, 92, 15–22. [Google Scholar] [CrossRef]
- Yu, J.W.; Jung, J.; Choi, Y.M.; Choi, J.H.; Yu, J.; Lee, J.K.; You, N.H.; Goh, M. Enhancement of the crosslink density, glass transition temperature, and strength of epoxy resin by using functionalized graphene oxide co-curing agents. Polym. Chem. 2016, 7, 36–43. [Google Scholar] [CrossRef]
- Sanes, J.; Saurin, N.; Carrion, F.J.; Ojados, G.; Bermudez, M.D. Synergy between single-walled carbon nanotubes and ionic liquid in epoxy resin nanocomposites. Compos. Part B Eng. 2016, 105, 149–159. [Google Scholar] [CrossRef]
- Li, W.B.; Shang, T.H.; Yang, W.G.; Yang, H.C.; Lin, S.; Jia, X.L.; Cai, Q.; Yang, X.P. Effectively exerting the reinforcement of dopamine reduced graphene oxide on epoxy-based composites via Strengthened Interfacial Bonding. ACS Appl. Mater. Interfaces 2016, 8, 13037–13050. [Google Scholar] [CrossRef] [PubMed]










| Sample | DGEBA | AGE | u-Silica | IL-Silica | 1-MI | CPTMS |
|---|---|---|---|---|---|---|
| silica20 | 100 | 15 | 14.46 | 0 | 1.62 | 3.92 |
| IL-silica20 | 100 | 15 | 0 | 20 | 0 | 0 |
| IL-silica30 | 100 | 15 | 0 | 30 | 0 | 0 |
| IL-silica40 | 100 | 15 | 0 | 40 | 0 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Mi, X.; Tian, J.; Zhang, J.; Xu, T. Supported Ionic Liquid Silica as Curing Agent for Epoxy Composites with Improved Mechanical and Thermal Properties. Polymers 2017, 9, 478. https://doi.org/10.3390/polym9100478
Zhang C, Mi X, Tian J, Zhang J, Xu T. Supported Ionic Liquid Silica as Curing Agent for Epoxy Composites with Improved Mechanical and Thermal Properties. Polymers. 2017; 9(10):478. https://doi.org/10.3390/polym9100478
Chicago/Turabian StyleZhang, Chongrui, Xiaoqian Mi, Junyu Tian, Junheng Zhang, and Tiwen Xu. 2017. "Supported Ionic Liquid Silica as Curing Agent for Epoxy Composites with Improved Mechanical and Thermal Properties" Polymers 9, no. 10: 478. https://doi.org/10.3390/polym9100478
APA StyleZhang, C., Mi, X., Tian, J., Zhang, J., & Xu, T. (2017). Supported Ionic Liquid Silica as Curing Agent for Epoxy Composites with Improved Mechanical and Thermal Properties. Polymers, 9(10), 478. https://doi.org/10.3390/polym9100478