1. Introduction
The four valves in the mammalian heart are responsible for controlling the one-way direction of the blood stream from the heart to body and vice versa [
1,
2]. For this purpose, the inlet and outlet valves open and close continuously during the cardiac cycles. The malfunction of a valve has resulted in blood circulation disorders and may cause serious heart disease and even death [
3]. Currently, when one of the valves malfunctions, the end step of medical choice is to replace it with an artificial one that is state of the art. The drawbacks of artificial valves (either mechanical or biological) such as infection, inflammation, thromboembolic, anticoagulation medication requirement and low durability compel the biomedical engineer to introduce a new concept of tissue engineering heart valve (TEHV) [
4]. TEHV is an advanced principle to develop a neo-valvular tissue that can mimic the original tissue characteristics with the capacity to grow, repair and remodel in vivo. Based on tissue engineering (TE) principle, a three-dimensional (3D) scaffold is fabricated using the biomaterials as the initial template for the cells. The shape, structure and mechanical properties of fabricated scaffold should resemble the original aortic heart valve [
2,
5]. Thus the utilized materials as well as fabrication technique can significantly influence the scaffold properties.
Electrospinning is a versatile method for fabricating high quality of continuous nanofibers. The nanofibers are accumulated over each other and shape the electrospun mats. The mats obtained have hierarchy structure that is suitable for scaffolding engineered tissue. The 3D scaffold is then seeded with proper source of cells and biological behaviors are investigated. The hierarchy structure of the scaffold facilitated the nutrient supply to the cells (particularly those far away from the surface) as well as waste removal. Later, the cell seeded 3D scaffold is developed in a bioreactor (in vitro) prior to implementation into the body (in vivo). Obtaining a proper structure and morphology of the nanofibers mats via electrospinning requires optimum setting for electrospinning parameters involved as well as solution parameters. Previously, initial evaluation of electrospinning process was performed as reported in several publications [
6,
7,
8,
9,
10]. In our latest publication [
10], the parameters involved such as flow rate, voltage, collector rotating speed and solution parameters such as concentration and ratio of compounds were evaluated to determine the process behavior in terms of mechanical strength. The analysis of results indicated significance and importance level of the investigated parameters. The maximum elastic modulus obtained was roughly 20 ± 1.2 MPa in such a way that elasticity was around 20%, which is not quite desirable for heart valve tissue engineering. It is expected that the scaffold may lose mechanical properties after cell seeding when the degradation rate starts (roughly 10–12 MPa loss is expected during one-month incubation of the scaffold in particular condition) [
11,
12,
13]. However, statistical analysis shows the optimum point is achievable somewhere near the selected range. For this purpose, mathematical techniques such as steepest ascent/descent and response surface methodology are useful to explore this region. These methods enable us to move around the previous experimental range to find the optimum point. Furthermore, a quadratic regression model will be obtained that can be used for further point prediction. The advantage of TE concept compared to the current prosthesis is the potential to mimic the original tissue and no further medication treatment, inflammation and reoperation during the long time is required.
A significant difference between the left and right side of the heart is that the left heart distributes blood to a wider part of the body, naturally achieving a peak pressure six times more than the right side. Subsequently, the mitral and aortic valves on the left side of the heart are exposed to much higher pressure than the pulmonary and tricuspid valves. The wall of the left side is thicker than the right side. Generally, the majority of valvular dysfunctions are related to the left heart’s valves [
14,
15,
16]. The aortic heart valve operates under a dynamic tensile–shear–flexural loading, and tolerates an elastic modulus within 10–15 MPa [
11,
17]. The heart pumps around 3–5 L blood with velocity around 1.35 ± 0.35 m/s every minute [
18]. The suggested value for shear stress value for aortic heart valve is approximately 1–8 Pa, while the peak of this value may increase to a range of 3–150 Pa. In heart valve function, elasticity as well as strength are the two undeniable characteristics [
19,
20].
Several uniaxial tests were performed on both human and animal aortic heart valves to recognize the mechanical behavior. The studies mainly reported an elastic modulus for aortic heart valve in circumferential and radial direction. The reports indicate a much weaker elastic modulus in radial direction compared to circumferential direction. This can be attributed to the direction of collagen fibers that are dispersed circumferentially along the leaflets. In addition, the comparison between human and common animal valves mechanical properties exhibited a much weaker behavior for animals. Balguid and Rubbens [
11] reported that the elastic modulus of native human aortic valve to be 15 MPa with ultimate tensile strength of 2.6 MPa and 22% of strain in circumferential direction. In the same report, the elastic modulus in radial direction was measured to be 2 MPa with ultimate tensile strength of 0.4 MPa and strain of 30%.
Table 1 lists the related works pertaining to the uniaxial tensile tests of human and animal aortic valve. The average required elastic modulus seems to be approximately 14.5 MPa with at least 22% of elasticity in circumferential direction.
Previously, various types of synthetic/natural biomaterials have been used for scaffolding the heart valve. The synthetic/polymeric based materials must be biocompatible, biodegradable and fulfill the required mechanical properties for the dynamic function of heart valve. Synthetic biodegradable polymers such as polyglycolic acid (PGA) [
24,
25], polycaprolactone (PCL) [
26,
27], polylactic acid (PLA) [
28,
29] , and polyglycerol sebacate (PGS) [
30,
31] have already been reported for TEHV. Sodian et al. [
32] engineered tissue scaffolds using PLA and PGA copolymers where the scaffolds obtained were thicker and less flexible than the individual polymers. Scaffolding PLA polymer has been reported by Armentano et al. [
29] and, despite the desired tensile strength, it failed during dynamic mechanism of heart valve leaflets due to its high rigidity (brittleness). Van Lieshout et al. [
33] reported the application of PCL in TEHV through the electrospinning technique. The biomechanical behavior was good but the low degradation rate of PCL (more than two years) is a hindrance. Masoumi et al. [
30] investigated the scaffolding of PGS in TEHV which demonstrates good biodegradability, stiffness and cell adhesion compared to PGA. The PGS tensile strength tests exhibited nonlinear stress–strain behavior. The average elastic modulus of PGS was within the range of 0.025–1.2 MPa, which was not sufficient for heart valve. The main advantage of a synthetic scaffold is the fact that biomechanics and degradation properties can be chemically controlled according to the requirements. Although no biodegradable polymeric materials have been proven to be a desirable substitute for the native valves, work continues to be promising [
1,
15,
24,
25,
26,
27,
28,
29].
In our previous study [
5], maghemite (γ-Fe
2O
3) loaded thermoplastic polyurethane (TPU)/poly-
l-lactic acid (PLLA) was used as a novel mixture for fabricating aortic heart valve’s engineered tissue. The presence of maghemite nanoparticles in the nanofibers latter’s surface improved both cell proliferation rate and also the mechanical properties [
10,
34]. The elastic modulus of the 50:50% (
v/v) neat TPU/PLLA was improved from 3.24 to 4.76 MPa (roughly 32%) when TPU/PLLA was impregnated with γ-Fe
2O
3. However, it is still far away from the required elastic modulus for a heart valve (around 10–15 MPa). Similarly, the cell viability improved from 72.12% to 95.41% during 72 h of incubation. The results indicated 50:50% TPU/PLLA containing 1% γ-Fe
2O
3 nanoparticles exhibited overall satisfaction in terms of structural, biological and mechanical properties. The Taguchi analysis was performed to determine the significant parameters influencing the behavior of the selected parameters and the linear regression model was obtained which can only justify the performed experimental range and cannot be generalized for every other process out of that range. In addition, the initial analysis represents half of the curvature (in 3D surface plot) within that range, which means the optimum point is somewhere out of that selected range. Therefore, in this research, the novelty lies in the utilization of initial evaluation and regression model to move from that range to near the optimum point and a second order regression model pertaining to elastic modulus and electrospinning process can be generalized. This was done using the steepest ascent and response surface methodology (RSM). In addition, the cell seeding over the 3D scaffold for 34 days is another novelty of this paper. Besides cell seeding, the new concept is roughness study by AFM techniques for preliminary cell attachment evaluation. Finally, the micro-indentation test was performed after cell seeding to elucidate the elastic modulus loss, which can be attributed to polymer degradation and other factors.
2. Materials and Methods
2.1. Materials
Almost all chemical used in this research were purchased and no further purification has been applied on them. Bio-grade PLLA granules (Mw 70 kDa, glass transition temperature (Tg) 55–60 °C) was purchased from Ingeo™ Biopolymer 4032D (Minnetonka, MN, USA). Bio-grade transparent TPU granules (Mw 90 kDa, Tg of −50 °C) was purchased from Wenzhou City Sanho Co., Ltd. (Wenzhou, China) The dichloromethane (DCM) and dimethylformamide (DMF), as the solvent for PLLA and TPU, respectively, were purchased from Merck, Co. (Johor Bahru, Malaysia).
The degradability of PLLA and TPU is already verified in terms of changes in the morphology, mass and porosity [
5,
35] where a 50:50% (TPU/PLLA) scaffold during 24 weeks of incubation in simulated body fluid (SBF), had lose 47.15% of its mass while the morphology showed breakage on the fibers and porosity was increased roughly 15%. The 15 mL of 50:50% TPU/PLLA (
v/v) (with 6.54 wt % of concentration) solution was prepared by mixing PLLA and TPU solutions using a magnetic stirrer for 5 h, at room temperature (25 °C) to get a homogeneous solution.
In addition the γ-Fe
2O
3 nanoparticles were synthesized in laboratory as described in our previous research [
36]. Briefly, co-precipitation method [
37] was used where ferrous and ferric chloride were combined in stoichiometric in an ammonium hydroxide solution by alkaline co-precipitation for 4 h. Magnetite (Fe
3O
4), which is a black and gelatinous precipitate, was obtained. Nitric acid was then used to acidify the precipitate. Isolate the precipitates using magnetic decantation. The solution of ferric nitrate was then used to oxidize it at 100 °C for transforming the solution into γ-Fe
2O
3. Citrate anion was then used to coat the γ-Fe
2O
3 nanoparticles to prevent agglomeration. The precipitate was washed with acetone and finally dispersed in water resulting in the final stable state γ-Fe
2O
3 with a pH of 7. The solution was then freeze dried for 48 h to obtain a soft powder to be dispersed in the TPU/PLLA mixture [
6].
2.2. Response Surface Methodology for Optimizing the Electrospinning Setup
RSM is a collection of statistical and mathematical model to analyze, optimize and model a process. Generally, the RSM is applied after initial analysis (brainstorming) and obtaining the influential parameters on the related process. RSM procedure contains two main steps of steepest ascent/descent and central composite design. The most common model, which is used to define the relationship between vital input parameters and measurable output, is the quadratic regression model, which can be expressed as Equation (1):
where
is the response; β
0, β
i, β
ii and β
ij are the regression coefficients to be determined; “
k” represents the number of the factors “
xi”; and “ε” is the statistical error.
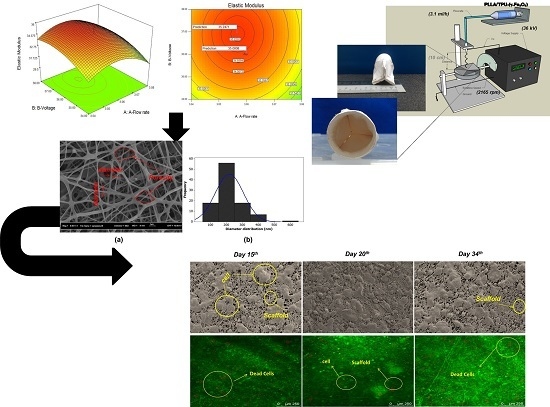
In electrospinning method, a high potential electrical field is applied on the emerging solution at the spinneret nozzle tip for transforming the jet into the nanofibers [
7,
8]. The flow rate of supplied solution was controlled through a syringe pump. The nanofibers were then deposited over the designed 3D collector. The process/system parameters involved such as flow rate (A), voltage (B), percentage of maghemite nanoparticles in the content (C) solution concentration (D) and collector rotating speed (E) were initially analyzed based on 2-level Taguchi experimental design in terms of elastic modulus [
9,
10] and then were optimized followed by the RSM. Briefly, the initial ANOVA analysis of Taguchi design exhibits the main parameters of voltage (B), percentage of maghemite nanoparticles in the content (C), solution concentration (D) and collector rotating speed (E) and two-way interaction of (BE) significantly effects on the elastic modulus of the nanofibers. The results indicated the parameters setting as flow rate (3 mL/h), voltage (30 kV), percentage of maghemite in the content (3%
w/v), solution concentration (10 wt %) and collector rotating speed (2000 rpm) were preferred for optimizing the elastic modulus of nanofibers within the applied experimental range where the elastic modulus of 28.49 ± 0.25 MPa was achieved. Based on the Taguchi results the best scaffold was obtained when the temperature was fixed at 25 °C and humidity was less than 10% (controlling the humidity using air-conditioner) [
10]. The needle–collector distance was fixed at 10 cm.
Usually, the primary approximation of the optimum operating condition for a process may be far away from the actual optimum. Therefore, in order to follow the optimization procedure the steepest ascent method with respect to the objective need to be applied. The steepest ascent objective is defined as exploring the vicinity of the current experimental region in order to achieve the most efficient direction towards the near optimum area. Using the steepest ascent, the minimum number of experiments would be designed to find the near optimum region. Usually, a linear regression model can fully justify the initial experimental condition. Basically, a perpendicular line to the gradient of the primary linear regression model (which is known as contours line) is the most efficient direction towards the optimum point. The Δ
xi is selected for one significant factor and correspondingly using the coefficients of the regression model; the Δ
xj is computed for the others one. The Δ
xi is given by Equation (2). The experiments were conducted according to the step size along the founded path of steepest ascent until no further increase in elastic modulus was obtained [
38,
39].
where β
i and β
j, are the regression coefficients for two significant factors, and Δ
xi and Δ
xj represent the step size for those factors, respectively.
2.3. Elastic Modulus of Electrospun Mats
To investigate the elastic modulus of electrospun mats the uniaxial tensile tests (according to standard testing method ASTM D882) were performed [
40]. Tensile strength test was done using universal testing machine (Lloyd, LRX, Singapore,) with a load cell of 0.5 kN at a cross-head speed of 5 mm/min. The electrospun mats consist of a strip of 12.7 mm wide by 76.2 mm in length and 0.3 ± 0.05 mm in thickness. The width of the specimen should not deviate by more than 2% over the length of the electrospun mats between the grips. The thickness of the specimens should not vary by more than 10% over the entire sample. The tests were performed at room temperature (25 °C) in three replicates. The specimens were dry (normal condition after spinning and no further modification was applied prior to testing) during the initial testing.
Elastic modulus (which is a measurement of the materials elasticity) was calculated using Equation (3) [
41,
42]:
where the “
” is the elastic modulus,
is the tensile stress and “
” the tensile strain.
2.4. Design of the 3D Aortic Heart Valve’s Template
To design a 3D template to be used as the collector, an aluminum foil was used. The required dimensions of aortic heart valve were extracted from previous works [
43,
44,
45].
Figure 1 illustrates the procedure of 3D template fabrication. The aluminum foil was cut into the leaflets shape (
Figure 1a), formed to the closed position of the valve by folding the free-edge length from the center (
Figure 1b) and attached to a foil covered cylindrical tube (
Figure 1c) [
46]. Normal plastic tape was used to attach the parts together. Later the template was attached as the spinning collector and based on the optimum settings the nanofibers were electrospun over the template. Finally, the aluminum foil was removed from the accumulated nanofibers over that (scaffold). In this design, the 3D scaffold was fabricated easily via one-step.
2.5. Structural, Porosity and Surface Roughness Properties
The morphology and diameter distribution of TPU/PLLA-(γ-Fe2O3) nanofibers were characterized using field emission scanning electron microscopy (FE-SEM, SUPRA 35VP, Oberkochen, Germany) with an accelerating voltage of 10.00 kV. The samples were cut into square shape with dimension 1 × 1 cm2. Prior the observation, the samples were coated using a gold sputter coater for 2 min. The diameter distribution of TPU/PLLA-(γ-Fe2O3) nanofibers was measured using image software (AxioVision Rel 4.8, Oberkochen, Germany). Hundred fibers of each sample were made to determine the average nanofiber diameter. The fibers were selected by the software randomly to prevent the bias. Furthermore, transmission electron microscopy (TEM, JEOL-JSM 6390 L, Chicago, IL, USA) was used for checking the dispersion of maghemite nanoparticles along the latter’s surface of the nanofibers.
The porosity of the 3D scaffold was measured through liquid displacement technique. In this method, a graduated cylindrical bottle was filled with a certain volume of ethanol (
V1). The scaffold was immersed into the bottle for 2 h until it became saturated with ethanol. The volume of ethanol was recorded as (
V2). The electrospun mats was then removed from ethanol and the remained volume of ethanol inside the bottle was recorded as (
V3). The porosity (%) of 3D scaffold was calculated using Equation (4):
where the “
V1” is the initial volume of ethanol, “
V2” is the volume of ethanol after scaffold immersion, and “
V3” is the volume of ethanol after scaffold removal.
The surface roughness of the electrospun mats was tested using the atomic force microscopy (AFM, 4-sided casted pyramidal tip, Park XE-100, Singapore). For this purpose, the electrospun mats were cut into 0.5 cm × 0.5 cm and the contact mode was utilized. The dimension of the scanning area was 10 µm × 10 µm (the scan angle was fixed at 0.00° and scan rate was at 10 Hz). The quantitative measurement of region statistics was performed, where the “Ra”, “Rq”, and “Rpv” represent the average surface roughness, root mean square surface roughness, and the peak-to-valley roughness of the scanned region, respectively. The higher surface roughness has resulted in better cell attachment.
2.6. Cells Viability and Attachment
The aortic smooth muscle cell (AOSMCs) was purchased from Sigma Aldrich
™ (St. Louis, MO, USA) and cultured based on normal culturing procedure up to passage 7. Briefly, three steps of thawing, plating and sub-culturing of the cells were performed. Initially, a cryopreserved vial of AOSMCs was removed from liquid nitrogen (LN
2) tank and the cells were thawed by placing the lower half of the vial in water bath at 37 °C for less than 2 min. Next, the cells suspension was pipetted into a centrifuge tube which was already filled by 9 mL of Dulbecco’s modified Eagle’s medium (DMEM) supplemented by 10% (
v/v) fetal bovine serum (FBS) and 1% (
v/v) penicillin. The solution was centrifuged at 1100 rpm for 5 min and correspondingly the cells deposited at the bottom of the tube were collected. The supernatant solution was removed and 1 mL of fresh DMEM was added to the tube. The tube was agitated gently to break the pellet and again disperse the cells. The cells suspension was pipetted to a T-75 flask containing 15 mL of DMEM (ratio of medium to surface area at 1:5 mL/cm
2). The T-75 flask was maintained in a 37 °C, 5% CO
2 humidified incubator. Every 24 h, the cell confluency was checked and, whenever the cells reached 80% confluency, sub-culturing was applied. To sub-culture the cells, the medium of T-75 flask was aspirated into the waste. Phosphate buffered saline (PBS, 3 mL) was pipetted into the flask and agitated smoothly to wash the monolayer of the cells and remove the remaining DMEM. After washing, the PBS was aspirated and 3 mL of trypsin-inhibitor was poured into the flask (in order to detach the cells from the bottom of flask) and incubated at 37 °C, 5% CO
2 humidified incubator for 3–5 min. Afterwards, the flask was removed from incubator and the solution (containing trypsin and detached cells) was pipetted to a centrifuge tube that was already filled by 5 mL of DMEM. The solution was centrifuged at 980 rpm for 5 min to pellet the cells. The supernatant solution was aspirated and 2 mL of fresh DMEM was added to the tube. The tube tip was flicked gently to break up the cells clumps and resuspends the cell. Finally, two T-75 flasks containing 15 mL of DMEM were prepared and 1 mL of cells suspension was pipetted into each flask. The flasks were maintained at 37 °C, 5% CO
2 humidified incubator. This procedure was repeated up to reach passage 7 of the cells. Once the cells were trypsinized one passage was counted. Suitable number of cells (5 × 10
4 cell/cm
2) was then seeded over the scaffold loaded into a 50 mL centrifuge tube. The qualitative and quantitative analysis of cells attachment and proliferation were performed using confocal laser microscopy (CLM, Leica-TCS, SP8, Wetzlar, Germany) and MTT assay, respectively, during 15th, 20th and 34th days of incubation. To observe the cells proliferation and attachment, acridine orange/ethidium bromide (AO/EB) staining assay with an excitation maximum at 502 nm and an emission maximum at 525 nm (green) (while in association with RNA the emission maximum shift to 620 nm) was used in such a way that in every mentioned day, the medium was removed and the scaffold was washed with phosphate buffer saline (PBS) twice. The scaffold was then incubated in medium culture supplemented by 80 µL of AO/EB (10 µM) for 20 min. Later, the scaffold was washed with PBS to remove the dye and observed under the CLM within wavelength of 520–610 nm [
47].
Furthermore, to measure the cell density, the MTT assay procedure was done. For this purpose, in every mentioned period, the scaffold was removed from the tube and washed twice with PBS and cut longitudinally. The sample was plated in well plate supplemented by 5 mL DMEM and later 80 µL of MTT reagent (5 mg/mL in PBS) was added to the plate and incubated in dark area for 4 h at 37 °C and 5% CO
2. Then, process was continued by removing the solution from plates and adding DMSO (1 mL) to dissolve the formazan crystals. In addition, similar procedure was done for the tube containing no scaffold (control plate). Then, 100 µL of the solutions were pipetted into a 96- well plate and the cell viability% using Elisa absorbance reader (BioTek, ELx808, Winooski, VT, USA) was measure at 570 nm of wavelength using Equation (5):
where “
Ds” is the absorbance value of the scaffold and “
Dc” is the absorbance value of control.
2.7. Macro-Indentation Test
Macro-indentation flexural test through LF plus materials testing analyzer apparatus (Lloyd Tensile Tester, EZ, Singapore) according to the standard ISO 14577-1 was performed to analyze the deformation and elastic modulus loss percent of scaffold during 15th, 20th and 34th days of incubation after cell seeding. The elastic modulus measurements via macro-indentation bending test was performed easily as compared to very small elongation in compressive or tensile load. To prepare the samples for the test, the scaffold was removed from culture medium at each time point and the leaflets were separated from the root. Three leaflets were cut from commissures and clamped over a round hole with diameter 21 mm with a ball probe (diameter 10 mm). Indentation force of 100 N at quasi-static rate at a cross head speed of 10 mm/min was applied to leaflets until rupture occurred (rupture is defined as a sudden 50% loss in load). To analyze the elastic modulus, the maximum load that can be applied to the leaflets before rupture (N) and maximum extension at break (indentation depth before rupture) need to be translated into stress and strain. The results were analyzed through the applied load versus the depth of indentation by NexygenPlus data analysis software, Unites States. The tests were performed three times (n = 3) in two groups of wet and dried scaffolds.
The Hertz theory was used to analyze the force indentation data [
48,
49]. Equations (6) and (7) represent the procedure for calculating the elastic modulus of scaffold. The relative elastic modulus “
Er” is given by:
where “
F” represents the force applied, “δ” is the depth of indentation, and “
Re” is the contact surface area between ball probe and leaflet.
The elastic modulus of leaflets (electrospun nanofibers)
Ee was calculated by;
where
Vf, is the Poisson’s ratio of the nanofibers which is assumed as 0.33 [
49].
In addition, the bending stiffness of the scaffold was calculated through rupture test. The force was applied to the specimen until the rupture occurred. The linear beam theory (Equation (8)) was used to calculate the stiffness (N·mm
2).
where “
EI” represents the stiffness index, “
F” is the maximum applied force before rupture, “
L” is the span length between grids, and “
” represents the maximum depth of indentation [
50,
51].