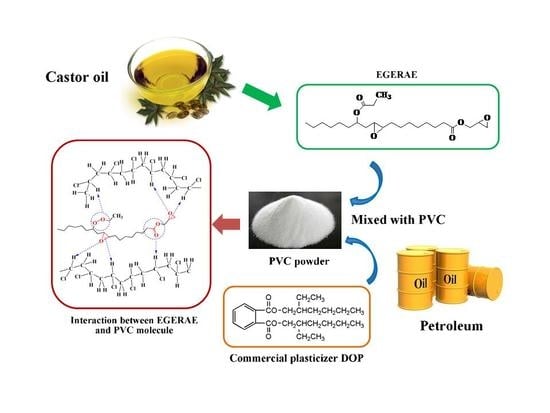
Synthesis and Properties of a Novel Environmental Epoxidized Glycidyl Ester of Ricinoleic Acetic Ester Plasticizer for Poly(vinyl chloride)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RAE
2.3. Preparation of GERAE
2.4. Preparation of EGERAE
2.5. Preparation of Plasticized PVC Test Specimens
2.6. Characterizations
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Thermal Stability
3.3. Dynamic Mechanical Analysis
3.4. Mechanical Properties
3.5. Exudation, Volatility and Extraction Resistance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vieira, M.G.A.; Da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Tullo, A.H. Plastics additives' steady evolution. Chem. Eng. News 2000, 78, 21–31. [Google Scholar] [CrossRef]
- Bocqué, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.-J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym. Sci. A 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Shipley, S.; Börmann, A.; Nicell, J.A.; Maric, M.; Leask, R.L. Designing green plasticizers: Influence of molecule geometry and alkyl chain length on the plasticizing effectiveness of diester plasticizers in PVC blends. Polymer 2016, 89, 18–27. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; Da Silva, M.A.; Maçumoto, A.C.G.; Dos Santos, L.O.; Beppu, M.M. Synthesis and application of natural polymeric plasticizer obtained through polyesterification of rice fatty Acid. Mater. Res. 2014, 17, 386–391. [Google Scholar] [CrossRef]
- Stevenswrited, M. Polymer Chemistry: An Introduction, 3rd ed.; Addison-Wesley Publishing Company Inc.: Boston, MA, USA, 1999; p. 30. [Google Scholar]
- Drake, P.L.; Rojas, M.; Reh, C.M.; Mueller, C.A.; Jenkins, F.M. Occupational exposure to airborne mercury during gold mining operations near El Callao, Venezuela. Int. Arch. Occup. Environ. Health 2001, 74, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.; De Korte, D. DEHP-plasticised PVC: Relevance to blood services. Transfus. Med. 2011, 21, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, F.; Ferri, M.; Morelli, A.; Dipaola, L.; Latini, G. Perspectives on alternatives to phthalate plasticized poly(vinyl chloride) in medical devices applications. Prog. Polym. Sci. 2013, 38, 1067–1088. [Google Scholar] [CrossRef]
- Chiellini, F.; Ferri, M.; Latini, G. Physical-chemical assessment of di-(2-ethylhexyl)-phthalate leakage from poly(vinyl chloride) endotracheal tubes after application in high risk newborns. Int. J. Pharm. 2011, 409, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.T.; Carrier, D.J.; Hwang, B.J.; Licence, P.; Moores, A.; Pradeep, T.; Sels, B.; Subramaniam, B.; Tam, M.K.C.; Williams, R.M. Four years of ACS sustainable chemistry & engineering: Reflections and new developments. ACS Sustain. Chem. Eng. 2017, 5, 1–2. [Google Scholar]
- Yang, D.; Peng, X.; Zhong, L.; Cao, X.; Chen, W.; Sun, R. Effects of pretreatments on crystalline properties and morphology of cellulose nanocrystals. Cellulose 2013, 20, 2427–2437. [Google Scholar] [CrossRef]
- Fernandez, S.S.; Kunchandy, S.; Ghosh, S. Linseed oil plasticizer based natural rubber/expandable graphite vulcanizates: Synthesis and characterizations. J. Polym. Environ. 2015, 23, 1–8. [Google Scholar] [CrossRef]
- Caes, B.R.; Teixeira, R.E.; Knapp, K.G.; Raines, R.T. Biomass to furanics: Renewable routes to chemicals and fuels. ACS Sustain. Chem. Eng. 2016, 3, 2591–2605. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Chrissafis, K.; Exarhopoulos, S.; Bikiaris, D.N. Furan-based polyesters from renewable resources: Crystallization and thermal degradation behavior of poly(hexamethylene 2,5-furan-dicarboxylate). Eur. Polym. J. 2015, 67, 383–396. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilization of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Uyama, H.; Kuwabara, M.; Tsujimoto, T.; Kobayashi, S. Enzymatic synthesis and curing of biodegradable epoxide-containing polyesters from renewable resources. Biomacromolecule 2003, 4, 211. [Google Scholar] [CrossRef] [PubMed]
- Noel, A.; Borguet, Y.P.; Wooley, K.L. Self-reporting degradable fluorescent grafted copolymer micelles derived from biorenewable resources. ACS Macro Lett. 2015, 4, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chang, P.R.; Ma, X. Preparation and properties of layered double hydroxide–carboxymethylcellulose sodium/glycerol plasticized starch nanocomposites. Carbohydr. Polym. 2011, 86, 877–882. [Google Scholar] [CrossRef]
- Yang, D.; Peng, X.; Zhong, L.; Cao, X.; Chen, W.; Zhang, X.; Liu, S.; Sun, R. “Green” films from renewable resources: Properties of epoxidized soybean oil plasticized ethyl cellulose films. Carbohydr. Polym. 2014, 103, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Chavan, A.P.; Gogate, P.R. Ultrasound assisted synthesis of epoxidized sunflower oil and application as plasticizer. J. Ind. Eng. Chem. 2015, 21, 842–850. [Google Scholar] [CrossRef]
- Mehta, B.; Kathalewar, M.; Sabnis, A. Benzyl ester of dehydrated castor oil fatty acid as plasticizer for poly(vinyl chloride). Polym. Int. 2014, 63, 1456–1464. [Google Scholar] [CrossRef]
- Mehta, B.; Kathalewar, M.; Sabnis, A. Diester based on castor oil fatty acid as plasticizer for poly(vinyl chloride). J. Appl. Polym. Sci. 2014, 131, 2928–2935. [Google Scholar] [CrossRef]
- Feng, G.; Jia, P.; Zhang, L.; Hu, L.; Zhang, M.; Zhou, Y. Synthesis of a novel phosphorus-containing plasticizer based on castor oil and its application for flame retardancy of polyvinyl chloride. Korean J. Chem. Eng. 2015, 32, 1201–1206. [Google Scholar] [CrossRef]
- Jia, P.; Zhang, M.; Hu, L.; Feng, G.; Bo, C.; Zhou, Y. Synthesis and application of novel environmental castor oil based polyol ester plasticizers for poly(vinyl chloride). ACS Sustain. Chem. Eng. 2015, 3, 2187–2193. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Xia, J.; Ding, C.; Wang, M.; Xu, L.; Yang, X.; Huang, K. Tung oil based plasticizer and auxiliary stabilizer for poly(vinyl chloride). Mater. Des. 2017, 122, 366–375. [Google Scholar] [CrossRef]
- Pitchaimari, G.; Sarma, K.S.S.; Varshney, L.; Vijayakumar, C.T. Influence of the reactive diluent on electron-beam curable funtionalized N-(4-hydroxyl phenyl) maleimide derivatives—Studies on thermal degradation kinetics using model free approach. Thermochim. Acta 2014, 597, 8–18. [Google Scholar] [CrossRef]
- Yao, Q.; Wilkie, C.A. Thermal degradation of PVC in the presence of polystyrene. J. Vinyl Addit. Technol. 2001, 7, 26–36. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Wang, Y.; Huang, J.; Li, K.; Nie, X.; Jiang, J. Synthesis and application of environmental soybean oil based epoxidized glycidyl ester plasticizer for poly(vinyl chloride). Eur. J. Lipid Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Greco, A.; Brunetti, D.; Renna, G.; Mele, G.; Maffezzoli, A. Plasticizer for poly(vinyl chloride) from cardanol as a renewable resource material. Polym. Degrad. Stab. 2010, 95, 2169–2174. [Google Scholar] [CrossRef]
- Lai, H.; Wang, Z.; Wu, P.; Chaudhary, B.I.; Sengupta, S.S.; Cogen, J.M.; Li, B. Structure and diffusion behavior of Trioctyl Trimellitate (TOTM) in PVC film studied by ATR-IR spectroscopy. Ind. Eng. Chem. Res. 2012, 51, 9365–9375. [Google Scholar] [CrossRef]
- Soudais, Y.; Moga, L.; Blazek, J.; Lemort, F. Coupled DTA-TGA-FT-IR investigation of pyrolytic decomposition of EVA, PVC and cellulose. J. Anal. Appl. Pyrolysis 2007, 78, 46–57. [Google Scholar] [CrossRef]
- Linde, E.; Gedde, U.W. Plasticizer migration from PVC cable insulation—The challenges of extrapolation methods. Polym. Degrad. Stab. 2014, 101, 24–31. [Google Scholar] [CrossRef]
- Daniels, P.H. A brief overview of theories of PVC plasticization and methods used to evaluate PVC-plasticizer interaction. J. Vinyl Addit. Technol. 2009, 15, 219–223. [Google Scholar] [CrossRef]
- Vadukumpully, S.; Paul, J.; Mahanta, N.; Valiyaveettil, S. Flexible conductive graphene/poly(vinyl chloride) composite thin films with high mechanical strength and thermal stability. Carbon 2011, 49, 198–205. [Google Scholar] [CrossRef]









| Component (phr) | F0 | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|
| Total plasticizer content | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| EGERAE content | 0.0 | 10.0 | 20.0 | 30.0 | 40.0 |
| DOP content | 40.0 | 30.0 | 20.0 | 10.0 | 0.0 |
| EFAME content | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Thermal stabilizers content | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Sample | Tg (°C) | Ti (°C) | T10 (°C) | T50 (°C) | R300 (%) | R400 (%) |
|---|---|---|---|---|---|---|
| F0 | 41.5 | 262.2 | 255.5 | 300.5 | 49.6 | 27.9 |
| F1 | 39.2 | 263.7 | 265.7 | 310.7 | 59.6 | 30.5 |
| F2 | 46.9 | 261.6 | 268.2 | 313.2 | 60.6 | 34.1 |
| F3 | 53.1 | 267.1 | 273.1 | 320.6 | 66.8 | 35.4 |
| F4 | 51.0 | 271.8 | 275.8 | 320.8 | 68.7 | 35.8 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Modulus of Elasticity (MPa) |
|---|---|---|---|
| F0 | 5.0 ± 0.38 | 256.9 ± 23.77 | 2.4 ± 0.09 |
| F1 | 5.6 ± 0.32 | 318.8 ± 20.27 | 2.5 ± 0.24 |
| F2 | 6.0 ± 0.16 | 337.9 ± 21.47 | 9.6 ± 4.78 |
| F3 | 5.9 ± 0.55 | 308.4 ± 19.59 | 27.5 ± 1.76 |
| F4 | 6.8 ± 0.59 | 264.7 ± 13.40 | 57.5 ± 6.97 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, K.; Wang, Y.; Huang, J.; Nie, X.; Jiang, J. Synthesis and Properties of a Novel Environmental Epoxidized Glycidyl Ester of Ricinoleic Acetic Ester Plasticizer for Poly(vinyl chloride). Polymers 2017, 9, 640. https://doi.org/10.3390/polym9120640
Chen J, Li K, Wang Y, Huang J, Nie X, Jiang J. Synthesis and Properties of a Novel Environmental Epoxidized Glycidyl Ester of Ricinoleic Acetic Ester Plasticizer for Poly(vinyl chloride). Polymers. 2017; 9(12):640. https://doi.org/10.3390/polym9120640
Chicago/Turabian StyleChen, Jie, Ke Li, Yigang Wang, Jinrui Huang, Xiaoan Nie, and Jianchun Jiang. 2017. "Synthesis and Properties of a Novel Environmental Epoxidized Glycidyl Ester of Ricinoleic Acetic Ester Plasticizer for Poly(vinyl chloride)" Polymers 9, no. 12: 640. https://doi.org/10.3390/polym9120640
APA StyleChen, J., Li, K., Wang, Y., Huang, J., Nie, X., & Jiang, J. (2017). Synthesis and Properties of a Novel Environmental Epoxidized Glycidyl Ester of Ricinoleic Acetic Ester Plasticizer for Poly(vinyl chloride). Polymers, 9(12), 640. https://doi.org/10.3390/polym9120640