Effect of Zinc Oxide Modified Silica Particles on the Molecular Dynamics of Carboxylated Acrylonitrile-Butadiene Rubber Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Rubber Blends and Vulcanizates
2.3. Methods of Testing
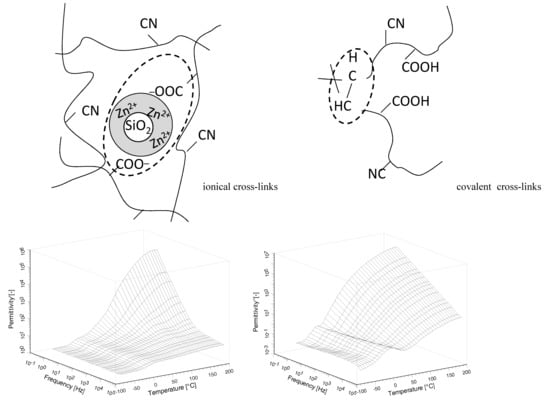
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- López-Manchado, M.A.; Valentin, J.L.; Carretero, J.; Barroso, F.; Arroyo, M. Rubber network in elastomer nanocomposites. Eur. Polym. J. 2007, 43, 4143–4150. [Google Scholar] [CrossRef]
- Ohbi, D.S.; Purewal, T.S.; Shah, T.; Siores, E. Crosslinking reaction mechanism of diisopropyl xanthogen polysulfide accelerator in bromobutyl elastomer for medical device applications. J. Appl. Polym. Sci. 2008, 107, 4013–4020. [Google Scholar] [CrossRef]
- Alam, N.; Mandal, S.K.; Debnath, S.C. Bis(N-benzyl-piperazine)thiuram disulfide and dibenzothiazyl disulfide as synergistic safe accelerators in the vulcanization of natural rubber. J. Appl. Polym. Sci. 2012, 126, 1830–1836. [Google Scholar] [CrossRef]
- Przybyszewska, M.; Zaborski, M.; Jakubowski, B.; Zawadiak, J. Zinc chelates as new activators for sulphur vulcanization of acrylonitrile-butadiene elastomers. Express Polym. Lett. 2009, 3, 256–266. [Google Scholar] [CrossRef] [Green Version]
- Naskar, K.; Noordermeer, J.W.M. Multifunctional peroxides as a means to improve properties of dynamically vulcanized PP/EPDM blends. Kautsch. Gummi Kunstst. 2004, 57, 235–239. [Google Scholar]
- Owczarek, M.; Zaborski, M. Chlorosulfonated polyethylene cross-linked with aminosilanes. Kautsch. Gummi Kunstst. 2005, 58, 432–437. [Google Scholar]
- Bhowmick, A.K.; Stephens, H.L. Handbook of Elastomers, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 2001; pp. 565–567. ISBN 9780824703837. [Google Scholar]
- Ibarra, L.; Alzorriz, M. Ionic elastomers based on carboxylated nitrile rubber and magnesium oxide. J. Appl. Polym. Sci. 2007, 103, 1894–1899. [Google Scholar] [CrossRef]
- Ibarra, L.; Rodrigues, A.; Mora, I. Ionic nanocomposites based on XNBR-OMg filled with layered nanoclays. Eur. Polym. J. 2007, 43, 753–761. [Google Scholar] [CrossRef]
- Ibarra, L.; Rodrigues, A.; Mora-Barrantes, I. Crosslinking of carboxylated nitrile rubber (XNBR) induced by coordination with anhydrous copper sulfate. Polym. Int. 2009, 58, 218–226. [Google Scholar] [CrossRef]
- Ibarra, L.; Rodrigues, A.; Mora-Barrantes, I. Crosslinking of unfilled carboxylated nitrile rubber with different systems: Influence on properties. J. Appl. Polym. Sci. 2008, 108, 2197–2205. [Google Scholar] [CrossRef]
- Tobolski, A.V.; Lyons, P.F.; Hata, N. Ionic clusters in high-strength carboxylic rubbers. Macromolecules 1968, 1, 515–519. [Google Scholar] [CrossRef]
- Eisenberg, A. Clustering of ions in organic polymers. A theoretical approach. Macromolecules 1970, 3, 147–154. [Google Scholar] [CrossRef]
- Mora-Barantes, I.; Malmierca, M.A.; Valentin, J.L.; Rodriguez, A.; Ibarra, L. Effect of covalent cross-links on the network structure of thermo-reversible ionic elastomers. Soft Matter. 2012, 8, 5201–5213. [Google Scholar] [CrossRef]
- De Luca, M.A.; Jacobi, M.M.; Orlandini, L.F. Synthesis and characterization of elastomeric composites prepared from epoxydized styrene butadiene rubber, 3-aminopropyltriethoxysilane and tetraethoxysilane. J. Sol-Gel Sci. Technol. 2009, 49, 150–158. [Google Scholar] [CrossRef]
- Chokanandsombad, Y.; Sirisinha, C. MgO and ZnO as reinforcing fillers in cured polychloroprene rubber. J. Appl. Polym. Sci. 2013, 128, 2533–2540. [Google Scholar] [CrossRef]
- Przybyszewska, M.; Zaborski, M. The effect of zinc oxide nanoparticle morphology on activity in crosslinking of carboxylated nitrile elastomers. Express Polym. Lett. 2009, 3, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Gaca, M.; Zaborski, M. The properties of elastomers obtained with the use of carboxylated acrylonitrile-butadiene rubber and new crosslinking substances. Polimery 2016, 61, 31–38. [Google Scholar] [CrossRef]
- Pietrasik, J.; Gaca, M.; Zaborski, M.; Okrasa, L.; Boiteux, G.; Gain, O. Studies of molecular dynamics of carboxylated acrylonitrile-butadiene rubber composites containing in situ synthesized silica particles. Eur. Polym. J. 2009, 45, 3317–3325. [Google Scholar] [CrossRef]
- Czech, P.; Okrasa, L.; Ulanski, J.; Boiteux, G.; Mechin, F.; Cassagnau, P. Studies of the molecular dynamics in polyurethane networks with hyperbranched crosslinkers of different coordination numbers. J. Appl. Polym. Sci. 2007, 105, 89–98. [Google Scholar] [CrossRef]
- Owczarek, M.; Zaborski, M.; Paryjczak, T.; Boiteux, G.; Gain, O. New type of inorganic filler with a core-shell structure. Macromol. Symp. 2003, 194, 313–319. [Google Scholar] [CrossRef]
- Owczarek, M.; Zaborski, M. Chlorosulfonated polyethylene elastomers containing zinc oxide incorporated on SiO2. Kautsch. Gummi Kunstst. 2004, 57, 218–223. [Google Scholar]
- Chen, Y.; Xu, C. Crosslink network evolution of nature rubber/zinc dimethacrylate composite during peroxide vulcanization. Polym. Compos. 2011, 32, 1505–1514. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C. Stress-strain behaviors and crosslinked networks studies of natural rubber-zinc dimethacrylate composites. J. Macromol. Sci. Part B 2012, 51, 1384–1400. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.; Zeng, X. A study on the crosslink network evolution of magnesium dimethacrylate/natural rubber composite. J. Appl. Polym. Sci. 2012, 125, 2449–2459. [Google Scholar] [CrossRef]
- Zaborski, M.; Owczarek, M.; Paryjczak, T.; Każmierczak, A. Właściwości karboksylowanego kauczuku butadienowo-akrylonitrylowego usieciowanego za pomocą układu ZnO/SiO2. Polimery 2002, 47, 339–346. [Google Scholar]
- Zaborski, M.; Paryjczak, T.; Kaźmierczak, A.; Albińska, J. Charakterystyka fizykochemicznych właściwości nieorganicznych składników mieszanin polimerowych o budowie jądro-powłoka. Polimery 2002, 47, 95–103. [Google Scholar]
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Shinichi, Y.; Kenji, T.; Nobuaki, N.; Shoichi, K.; Hitoshi, T.; Eisaku, H. Dielectric relaxation studies on water absorption of ethylene ionomers. Macromolecules 1992, 25, 7168–7171. [Google Scholar] [CrossRef]
- Vondracek, P.; Pouchaleon, A. Ammonia-induced tensile set and swelling in silica-filled silicone rubber. Rubb. Chem. Technol. 1990, 63, 202–214. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Springer: Berlin, Germany, 2003; pp. 2–13. ISBN 978-3-642-56120-7. [Google Scholar]
- Vidal, A.; Haidar, B. Filled elastomers: Characteristics and properties of interfaces and their role in reinforcement processes. Soft Mater. 2007, 5, 155–167. [Google Scholar] [CrossRef]
- Gaca, M.; Zaborski, M. The properties of ethylene–propylene elastomers obtained with the use of a new cross-linking substance. J. Term. Anal. Calorim. 2016, 125, 1105–1113. [Google Scholar] [CrossRef]









| Materials | Properties | Suppliers |
|---|---|---|
| Carboxylated butadiene-acrylonitrile rubber XNBR (trade name Krynac 7,5X) | 6.4% by weight of carboxylic groups; 26.3% by weight of acrylonitrile mers; density = 0.99 g/cm3 | Lanxess, Warsaw, Poland |
| Precipitated silica SiO2 (trade name Zeosil 175MP) | Surface area = 143 m2/g [26] | Solvay, Brussels, Belgium |
| Dicumyl peroxide (DCP) | Melting point = 39–41 °C | Fluka, Poznan, Poland |
| Zinc oxide (ZnO) | Density = 5.6 g/cm3 | Huta Bedzin, Bedzin, Poland |
| Modified silica particles (ZnO/SiO2) | Concentration of ZnO on the surface 1%–20% | Synthesized by the process discussed in reference [27] |
| Symbol of Sample | XP | XZ | XZS | X1mS | X8mS | X12mS | X20mS | |
|---|---|---|---|---|---|---|---|---|
| Compounds | ||||||||
| XNBR | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| DCP | 2 | - | - | - | - | - | - | |
| ZnO | - | 6 | 6 | - | - | - | - | |
| SiO2 | - | - | 30 | - | - | - | - | |
| 1ZnO/SiO2 | - | - | - | 30 | - | - | - | |
| 8ZnO/SiO2 | - | - | - | - | 30 | - | - | |
| 12ZnO/SiO2 | - | - | - | - | - | 30 | - | |
| 20ZnO/SiO2 | - | - | - | - | - | - | 30 | |
| Symbol of Sample | ν | ΔνA/ν (×)100% |
|---|---|---|
| XP | 7.70 | 2.30 |
| XZ | 3.91 | 10.4 |
| XZS | 5.11 | 24.7 |
| X1mS | 3.55 | 29.9 |
| X8mS | 4.08 | 38.0 |
| X12mS | 5.68 | 39.3 |
| X20mS | 7.21 | 46.1 |
| Compound | Tg (DSC) (°C) | Δcp (Jg−1K−1) | Tα (E″) (°C) | Tα (tan δ) (°C) | Tα′ (tan δ) (°C) | Tα′ − Tα (°C) | Height of tan δ at Tα | Height of tan δ at Tα′ | E′-75 (MPa) | E′20 (MPa) |
|---|---|---|---|---|---|---|---|---|---|---|
| XP | −19.2 | 0.25 | −12.7 | −4.1 | - | - | 1.52 | - | 2529.5 | 3.1 |
| XZ | −14.5 | 0.08 | −14.7 | −3.9 | 76.0 | 79.9 | 0.98 | 0.28 | 2710.2 | 7.7 |
| XZS | −15.8 | 0.14 | −13.3 | −2.7 | 63.0 | 65.8 | 0.98 | 0.27 | 4103.6 | 17.7 |
| X1mS | −19.7 | 0.01 | −12.6 | −1.8 | 129.6 | 131.4 | 0.87 | 0.30 | 2687.2 | 16.4 |
| X8mS | −18.7 | 0.09 | −15.6 | −4.8 | 158.9 | 163.7 | 0.92 | 0.32 | 4199.6 | 19.5 |
| X12mS | - | - | −14.1 | −3.0 | 59.8 | 62.9 | 0.94 | 0.22 | 2655.1 | 8.5 |
| X20mS | −17.7 | 0.11 | −13.3 | −2.2 | 63.9 | 66.1 | 0.89 | 0.25 | 2109.1 | 10.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaca, M.; Pietrasik, J.; Zaborski, M.; Okrasa, L.; Boiteux, G.; Gain, O. Effect of Zinc Oxide Modified Silica Particles on the Molecular Dynamics of Carboxylated Acrylonitrile-Butadiene Rubber Composites. Polymers 2017, 9, 645. https://doi.org/10.3390/polym9120645
Gaca M, Pietrasik J, Zaborski M, Okrasa L, Boiteux G, Gain O. Effect of Zinc Oxide Modified Silica Particles on the Molecular Dynamics of Carboxylated Acrylonitrile-Butadiene Rubber Composites. Polymers. 2017; 9(12):645. https://doi.org/10.3390/polym9120645
Chicago/Turabian StyleGaca, Magdalena, Joanna Pietrasik, Marian Zaborski, Lidia Okrasa, Gisèle Boiteux, and Olivier Gain. 2017. "Effect of Zinc Oxide Modified Silica Particles on the Molecular Dynamics of Carboxylated Acrylonitrile-Butadiene Rubber Composites" Polymers 9, no. 12: 645. https://doi.org/10.3390/polym9120645
APA StyleGaca, M., Pietrasik, J., Zaborski, M., Okrasa, L., Boiteux, G., & Gain, O. (2017). Effect of Zinc Oxide Modified Silica Particles on the Molecular Dynamics of Carboxylated Acrylonitrile-Butadiene Rubber Composites. Polymers, 9(12), 645. https://doi.org/10.3390/polym9120645