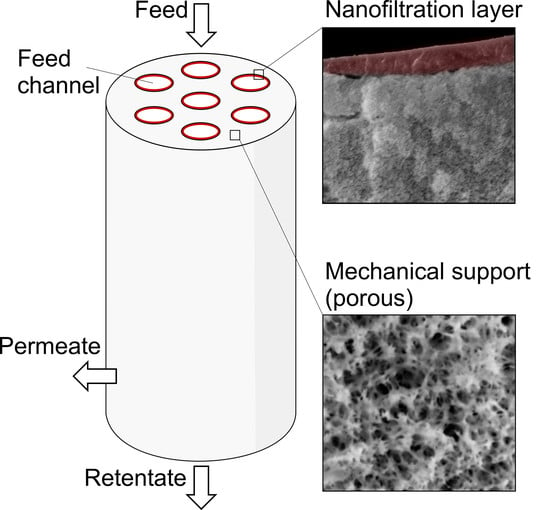
Poly(piperazine-amide)/PES Composite Multi-Channel Capillary Membranes for Low-Pressure Nanofiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Preparation of PES Multi-Channel Membranes
2.3. Preparation of Composite MCM
3. Results and Discussion
3.1. Support Membrane
3.2. Drying Time of Composite MCM
3.3. Contact Time of Monomer Solutions
3.4. Monomer Ratio
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, X.; Bian, X.; Shi, L. Preparation and characterization of NF composite membrane. J. Membr. Sci. 2002, 210, 3–11. [Google Scholar] [CrossRef]
- Benfer, S. Development and characterization of ceramic nanofiltration membranes. Sep. Purif. Technol. 2001, 22–23, 231–237. [Google Scholar] [CrossRef]
- Inopor GmbH. Ceramic Nanofiltration. Available online: http://www.inopor.com/en/products/ceramic-nanofiltration.html (accessed on 18 November 2017).
- Inge GmbH. Multibore® Membranes. Available online: http://www.inge.basf.com/ev/internet/inge/en/content/inge/Produkte/Multibore_Membran (accessed on 18 November 2017).
- Gille, D.; Czolkoss, W. Ultrafiltration with multi-bore membranes as seawater pre-treatment. Desalination 2005, 182, 301–307. [Google Scholar] [CrossRef]
- Bu-Rashid, K.A.; Czolkoss, W. Pilot Tests of Multibore UF Membrane at Addur SWRO Desalination Plant, Bahrain. Desalination 2007, 203, 229–242. [Google Scholar] [CrossRef]
- Heijnen, M.; Winkler, R.; Berg, P. Optimisation of the geometry of a polymeric Multibore® ultrafiltration membrane and its operational advantages over standard single bore fibres. Desalin. Water Treat. 2012, 42, 24–29. [Google Scholar] [CrossRef]
- GE Water. ZeeWeed 700B Membrane. Available online: https://www.suezwatertechnologies.com/products/zeeweed-700b-membrane (accessed on 18 November 2017).
- Wan, P.; Yin, J.; Deng, B. Seven-Bore Hollow Fiber Membrane (HFM) for Ultrafiltration (UF). Chem. Eng. Res. Des. 2017, 128, 240–247. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. Design and fabrication of lotus-root-like multi-bore hollow fiber membrane for direct contact membrane distillation. J. Membr. Sci. 2012, 421–422, 361–374. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.-S. Exploring the spinning and operations of multibore hollow fiber membranes for vacuum membrane distillation. AIChE J. 2014, 60, 1078–1090. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. A New-Generation Asymmetric Multi-Bore Hollow Fiber Membrane for Sustainable Water Production via Vacuum Membrane Distillation. Environ. Sci. Technol. 2013, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-J.; Zuo, J.; Chung, T.-S. Tri-bore PVDF hollow fibers with a super-hydrophobic coating for membrane distillation. J. Membr. Sci. 2016, 514, 165–175. [Google Scholar] [CrossRef]
- Bettahalli, N.S.; Lefers, R.; Fedoroff, N.; Leiknes, T.; Nunes, S.P. Triple-bore hollow fiber membrane contactor for liquid desiccant based air dehumidification. J. Membr. Sci. 2016, 514, 135–142. [Google Scholar] [CrossRef]
- Hua, D.; Ong, Y.K.; Wang, P.; Chung, T.-S. Thin-film composite tri-bore hollow fiber (TFC TbHF) membranes for isopropanol dehydration by pervaporation. J. Membr. Sci. 2014, 471, 155–167. [Google Scholar] [CrossRef]
- Luo, L.; Wang, P.; Zhang, S.; Han, G.; Chung, T.-S. Novel thin-film composite tri-bore hollow fiber membrane fabrication for forward osmosis. J. Membr. Sci. 2014, 461, 28–38. [Google Scholar] [CrossRef]
- Li, X.; Ang, W.L.; Liu, Y.; Chung, T.-S. Engineering design of outer-selective tribore hollow fiber membranes for forward osmosis and oil-water separation. AIChE J. 2015, 61, 4491–4501. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F.; Misdan, N.; Kassim, M.A. A recent progress in thin film composite membrane: A review. Desalination 2012, 287, 190–199. [Google Scholar] [CrossRef]
- Petersen, R.J. Composite reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1993, 83, 81–150. [Google Scholar] [CrossRef]
- Tomaschke, J.E. Membrane Preparation: Interfacial Composite Membranes. In Encyclopedia of Separation Science; Wilson, I.D., Adlard, E.R., Cooke, M., Poole, C.F., Eds.; Academic Press: Cambridge, MA, USA, 2000; pp. 3319–3331. [Google Scholar]
- Zhang, A.; Ma, R.; Xie, Y.; Xu, B.; Xia, S.; Gao, N. Preparation polyamide nanofiltration membrane by interfacial polymerization. Desalin. Water Treat. 2012, 37, 238–243. [Google Scholar] [CrossRef]
- Zhang, R.X.; Vanneste, J.; Poelmans, L.; Sotto, A.; Wang, X.L.; van der Bruggen, B. Effect of the manufacturing conditions on the structure and performance of thin-film composite membranes. J. Appl. Polym. Sci. 2012, 125, 3755–3769. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Rahimpour, A.; Peyravi, M. Developing thin film composite poly(piperazine-amide) and poly(vinyl-alcohol) nanofiltration membranes. Desalination 2010, 257, 129–136. [Google Scholar] [CrossRef]
- Wen, L.; Wang, W. Study on Compositions in Aqueous Phase of Hollow Fiber Composite Nanofiltration Membrane. Adv. Mater. Res. 2012, 529, 569–573. [Google Scholar] [CrossRef]
- Mansourpanah, Y.; Madaeni, S.; Rahimpour, A. Fabrication and development of interfacial polymerized thin-film composite nanofiltration membrane using different surfactants in organic phase; study of morphology and performance. J. Membr. Sci. 2009, 343, 219–228. [Google Scholar] [CrossRef]
- Song, Y.; Sun, P.; Henry, L.L.; Sun, B. Mechanisms of structure and performance controlled thin film composite membrane formation via interfacial polymerization process. J. Membr. Sci. 2005, 251, 67–79. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F. Progress in Interfacial Polymerization Technique on Composite Membrane Preparation. In Proceedings of the 2nd International Conference on Environmental Engineering and Applications, Shanghai, China, 19–21 August 2011; pp. 173–177. [Google Scholar]
- Setiawan, L.; Shi, L.; Wang, R. Dual layer composite nanofiltration hollow fiber membranes for low-pressure water softening. Polymer 2014, 55, 1367–1374. [Google Scholar] [CrossRef]
- Sun, S.P.; Hatton, T.A.; Chan, S.Y.; Chung, T.-S. Novel thin-film composite nanofiltration hollow fiber membranes with double repulsion for effective removal of emerging organic matters from water. J. Membr. Sci. 2012, 401–402, 152–162. [Google Scholar] [CrossRef]
- Fang, W.; Shi, L.; Wang, R. Interfacially polymerized composite nanofiltration hollow fiber membranes for low-pressure water softening. J. Membr. Sci. 2013, 430, 129–139. [Google Scholar] [CrossRef]
- Veríssimo, S.; Peinemann, K.V.; Bordado, J. Thin-film composite hollow fiber membranes: An optimized manufacturing method. J. Membr. Sci. 2005, 264, 48–55. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Yang, D.; Jian, X. Preparation and characterization of polypiperazine amide/PPESK hollow fiber composite nanofiltration membrane. J. Membr. Sci. 2007, 301, 85–92. [Google Scholar] [CrossRef]
- Frank, M.; Bargeman, G.; Zwijnenburg, A.; Wessling, M. Capillary hollow fiber nanofiltration membranes. Sep. Purif. Technol. 2001, 22–23, 499–506. [Google Scholar] [CrossRef]
- Veríssimo, S.; Peinemann, K.V.; Bordado, J. New composite hollow fiber membrane for nanofiltration. Desalination 2005, 184, 1–11. [Google Scholar] [CrossRef]
- Korikov, A.P.; Kosaraju, P.B.; Sirkar, K.K. Interfacially polymerized hydrophilic microporous thin film composite membranes on porous polypropylene hollow fibers and flat films. J. Membr. Sci. 2006, 279, 588–600. [Google Scholar] [CrossRef]
- Spruck, M.; Hoefer, G.; Fili, G.; Gleinser, D.; Ruech, A.; Schmidt-Baldassari, M.; Rupprich, M. Preparation and characterization of composite multichannel capillary membranes on the way to nanofiltration. Desalination 2013, 314, 28–33. [Google Scholar] [CrossRef]
- Qin, J.J.; Gu, J.; Chung, T.S. Effect of wet and dry-jet wet spinning on the shear-induced orientation during the formation of ultrafiltration hollow fiber membranes. J. Membr. Sci. 2001, 182, 57–75. [Google Scholar] [CrossRef]
- Rösler, H.W. Membrantechnolgie in der Prozessindustrie—Polymere Membranwerkstoffe. Chem. Ing. Tech. 2005, 77, 487–503. [Google Scholar] [CrossRef]
- Lui, J.; Xu, Z.L.; Li, X.H.; Zhang, Y.; Zhou, Y.; Wang, Z.X.; Wang, X.J. An improved process to prepare high separation performance PA/PVDF hollow fiber composite nanofiltration membranes. Sep. Purif. Technol. 2007, 58, 53–60. [Google Scholar] [CrossRef]
- Spruck, M.; Stadlmayr, W.; Koch, M.; Mayr, L.; Penner, S.; Rupprich, M. Influence of the coagulation medium on the performance of poly(ether sulfone) flat-sheet membranes. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Misdan, N.; Lau, W.J.; Ismail, A.F.; Matsuura, T.; Rana, D. Study on the thin film composite poly(piperazine-amide) nanofiltration membrane: Impacts of physicochemical properties of substrate on interfacial polymerization formation. Desalination 2014, 344, 198–205. [Google Scholar] [CrossRef]
- Paul, M.; Jons, S.D. Chemistry and fabrication of polymeric nanofiltration membranes: A review. Polymer 2016, 103, 417–456. [Google Scholar] [CrossRef]
- Zimmermann, R. Condensation Polymers: By Interfacial and Solution Methods. Angew. Chem. 1966, 78, 787. [Google Scholar] [CrossRef]
- Bartels, C.R.; Kreuz, K.L.; Wachtel, A. Structure-performance relationships of composite membranes: Porous support densification. J. Membr. Sci. 1987, 32, 291–312. [Google Scholar] [CrossRef]
- Freger, V. Swelling and Morphology of the Skin Layer of Polyamide Composite Membranes: An Atomic Force Microscopy Study. Environ. Sci. Technol. 2004, 38, 3168–3175. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M. Basic Principles of Membrane Technology; Springer: Dordrecht, The Netherlands, 1996; ISBN 978-94-009-1766-8. [Google Scholar]
- Dow Filmtec. NF90-400/34i Element. Available online: http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_0979/0901b80380979557.pdf (accessed on 18 November 2017).
- GE Water. Flat Sheet Membrane Chart. Available online: https://www.suezwatertechnologies.com/kcpguest/salesedge/documents/Technical%20Bulletins_Cust/Americas/English/TB1152EN.pdf (accessed on 18 November 2017).
- Synder Filtration. Sanitary Nanofiltration Spiral-Wound Element: NDX (500–700Da). Available online: http://synderfiltration.com/2014/wp-content/uploads/2017/04/NDX-Specsheet-TFC-500–700Da-Sanitary.pdf (accessed on 18 November 2017).
- Enkelmann, V.; Wegner, G. Mechanism of interfacial polycondensation and the direct synthesis of stable polyamide membranes. Makromol. Chem. 1976, 177, 3177–3189. [Google Scholar] [CrossRef]
- Liubimova, E.S.; Bildyukevich, A.V.; Melnikova, G.B.; Volkov, V.V. Modification of hollow fiber ultrafiltration membranes by interfacial polycondensation: Monomer ratio effect. Pet. Chem. 2015, 55, 795–802. [Google Scholar] [CrossRef]








| Parameter | Specification |
|---|---|
| Dope composition/wt % | PES/PD/PVP-K90/DMAc 17/15/5/63 |
| Dope flow rate/(cm3 min−1) | 68 |
| Dope temperature/°C | Room temperature |
| Bore fluid composition/wt % | Water/ethanol (80/20); pure water |
| Bore fluid flow rate/(L h−1) | 4.0 |
| Bore fluid temperature/°C | 30 ± 1 |
| Coagulation bath | Tap water |
| Coagulation bath temperature/°C | 30 ± 1 |
| Total spinning gap/cm | 15 |
| Steam gap/cm | 12 |
| Fibre post-treatment | 5000 ppm NaClO, three days |
| Step | Operation |
|---|---|
| 1 | Flushing with aqueous PIP/triethylamine (TEA) solution |
| 2 | N2 gas flushing for 6 min |
| 3 | Flushing with TMC in n-hexane solution |
| 4 | Post-treatment (drying) |
| ID | PIP | TMC | Ratio | t(PIP) | t(TMC) | R(MgSO4) | Flux | Rq |
|---|---|---|---|---|---|---|---|---|
| wt % | wt % | s | s | % | L m−2 h−1 | nm | ||
| BF W100 | - | - | - | - | - | 0.0 | 617.1 | 13.7 |
| NF 5 | 0.4 | 0.15 | 12.5 | 60 | 30 | 74.3 | 44.0 | - |
| BF W80E20 | - | - | - | - | - | 0.0 | 479.3 | 13.0 |
| NF2 | 0.4 | 0.15 | 12.5 | 60 | 30 | 90.5 | 34.4 | 15.5 |
| No. | t(PIP) | t(TMC) | R(MgSO4) | Flux |
|---|---|---|---|---|
| s | s | % | (L m−2 h−1) | |
| NF1 | 100 | 30 | 90.8 | 39.6 |
| NF2 | 60 | 30 | 90.5 | 34.4 |
| NF3 | 60 | 45 | 80.1 | 41.9 |
| NF4 | 60 | 60 | 88.6 | 42.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Back, J.O.; Spruck, M.; Koch, M.; Mayr, L.; Penner, S.; Rupprich, M. Poly(piperazine-amide)/PES Composite Multi-Channel Capillary Membranes for Low-Pressure Nanofiltration. Polymers 2017, 9, 654. https://doi.org/10.3390/polym9120654
Back JO, Spruck M, Koch M, Mayr L, Penner S, Rupprich M. Poly(piperazine-amide)/PES Composite Multi-Channel Capillary Membranes for Low-Pressure Nanofiltration. Polymers. 2017; 9(12):654. https://doi.org/10.3390/polym9120654
Chicago/Turabian StyleBack, Jan O., Martin Spruck, Marc Koch, Lukas Mayr, Simon Penner, and Marco Rupprich. 2017. "Poly(piperazine-amide)/PES Composite Multi-Channel Capillary Membranes for Low-Pressure Nanofiltration" Polymers 9, no. 12: 654. https://doi.org/10.3390/polym9120654
APA StyleBack, J. O., Spruck, M., Koch, M., Mayr, L., Penner, S., & Rupprich, M. (2017). Poly(piperazine-amide)/PES Composite Multi-Channel Capillary Membranes for Low-Pressure Nanofiltration. Polymers, 9(12), 654. https://doi.org/10.3390/polym9120654