Coating of TPU-PDMS-TMS on Polycotton Fabrics for Versatile Protection
Abstract
:1. Introduction
2. Experimental
2.1. Materials
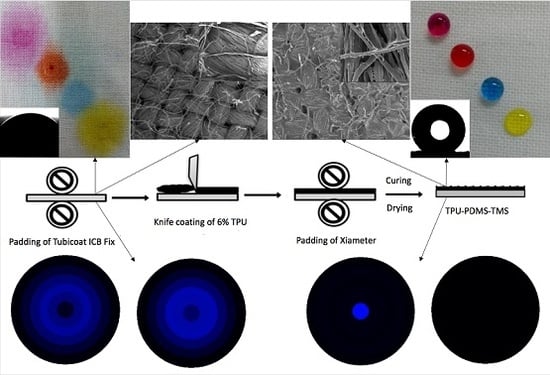
2.2. Methods
2.3. Measurements and Characterizations
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Water Contact Angle
2.3.4. Water Repellency
2.3.5. Oil Repellency
2.3.6. Aqueous Liquid Repellency
2.3.7. Chemical Resistance
2.3.8. Air Permeability
2.3.9. Laundering Test
2.3.10. Crocking Test
2.3.11. Thermal Resistance
2.3.12. Water Vapor Resistance
2.3.13. Moisture Management Property
2.3.14. Handle Property
3. Results and Discussion
3.1. Characterizations
3.1.1. FTIR Spectra
3.1.2. SEM Photos
3.2. Versatile Protection
3.2.1. Water Contact Angle
3.2.2. Water Repellency
3.2.3. Oil Repellency
3.2.4. Aqueous Liquid Repellency
3.2.5. Chemical Resistance
3.3. Comfort
3.3.1. Air Permeability
3.3.2. Water Vapor Resistance and Permeability
3.3.3. Thermal Resistance
3.3.4. Moisture Management Property
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pan, S.; Kota, A.K.; Mabry, J.M.; Tuteja, A. Superomniphobic surfaces for effective chemical shielding. J. Am. Chem. Soc. 2013, 135, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Gorzkowska-Sobas, A.A. Chemical Warfare Agents and Their Interactions with Solid Surfaces; Norwegian Defence Research Establishment (FFI): Kjeller, Norway, 2013. [Google Scholar]
- Jiang, W.-C.; Huang, Y.; Gu, G.; Meng, W.; Qing, F. A novel waterborne polyurethane containing short fluoroalkyl chains: Synthesis, characterization and its application on cotton fabrics surface. Appl. Surf. Sci. 2006, 253, 2304–2309. [Google Scholar] [CrossRef]
- Lim, H.; Lee, Y.; Park, I.J.; Lee, S.B. Synthesis and surface property of aqueous fluorine-containing polyurethane. J. Colloid Interface Sci. 2001, 241, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Hekster, F.M.; De Voogt, P.; Pijnenburg, A.M.C.M.; Laane, R.W.P.M. Perfluoroalkylated Substances: Aquatic Environmental Assessment; Rijkswaterstaat: Utrecht, The Netherlands, 2002. [Google Scholar]
- Daoud, W.A.; Xin, J.H.; Tao, X. Superhydrophobic Silica Nanocomposite Coating by a Low-Temperature Process. J. Am. Ceram. Soc. 2004, 87, 1782–1784. [Google Scholar] [CrossRef]
- Satam, D.; Lee, H.J.; Wilusz, E. An approach to mass customization of military uniforms using superoleophobic nonwoven fabrics. AATCC Rev. 2010, 10, 59–63. [Google Scholar]
- Roe, B.; Zhang, X. Durable hydrophobic textile fabric finishing using silica nanoparticles and mixed silanes. Text. Res. J. 2009, 79, 1115–1122. [Google Scholar] [CrossRef]
- Lomax, G.R. Breathable polyurethane membranes for textile and related industries. J. Mater. Chem. 2007, 17, 2775–2784. [Google Scholar] [CrossRef]
- Chen, R.S.; Chang, C.J.; Chang, Y.H. Study on siloxane-modified polyurethane dispersions from various polydimethylsiloxanes. J. Polym. Sci. A 2005, 43, 3482–3490. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Tian, X.; Cui, P.; Ding, X.; Ye, X. The effects of polydimethylsiloxane on transparent and hydrophobic waterborne polyurethane coatings containing polydimethylsiloxane. Phys. Chem. Chem. Phys. 2014, 16, 6787–6794. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, K. Chemical Protective Clothing; Wiley Online Library: Hoboken, NJ, USA, 1995. [Google Scholar]
- Walker, J.; Schreuder-Gibson, H.; Yeomans, W.; Hill, C. Development of Self-Decontaminating Textiles with Microporous Membranes; Army Natick Soldier Center: Natick, MA, USA, 2002. [Google Scholar]
- Jeong, W.Y.; An, S.K. The transport properties of polymer membrane-fabric composites. J. Mater. Sci. 2001, 36, 4797–4803. [Google Scholar] [CrossRef]
- Romaškevič, T.; Budrienė, S.; Pielichowski, K.; Pielichowski, J. Application of polyurethane-based materials for immobilization of enzymes and cells: A review. Chemija 2006, 17, 74–89. [Google Scholar]
- Kang, Y.K.; Park, C.H.; Kim, J.; Kang, T.J. Application of electrospun polyurethane web to breathable water-proof fabrics. Fibers Polym. 2007, 8, 564–570. [Google Scholar] [CrossRef]
- Makal, U.; Wood, L.; Ohman, D.E.; Wynne, K.J. Polyurethane biocidal polymeric surface modifier. Biomaterials 2006, 27, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, X.; Zhang, H.; Zhuang, X.; Yan, X. Facile dip-coating process towards multifunctional nonwovens: Robust noise reduction, abrasion resistance and antistastic electricity. Text. Res. J. 2017. [Google Scholar] [CrossRef]
- Su, X.; Li, H.; Lai, X.; Zhang, L.; Wang, J.; Liao, X.; Zeng, X. Vapor-liquid sol-gel approach to fabricating highly durable and robust superhydrophobic polydimethylsiloxane@silica surface on polymer textile for oil-water separation. ACS Appl. Mater. Interfaces 2017, 9, 28089–28099. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, F.; Periolatto, M.; Tempestini, L. Water and oil repellent finishing of textiles by UV curing: Evaluation of the influence of scaled-up process parameters. Coatings 2017, 7, 60. [Google Scholar] [CrossRef]
- Moiz, A.; Vijayan, A.; Padhye, R.; Wang, X. Chemical and water protective surface on cotton fabric by pad-knife-pad coating of WPU-PDMS-TMS. Cellulose 2016, 23, 3377–3388. [Google Scholar] [CrossRef]
- Arkles, B. Hydrophobicity, hydrophilicity and silanes. Paint Coat. Ind. 2006, 22, 114–135. [Google Scholar]
- Borisova, A.; Reihmane, S. Hydrophobic treatment of blended fabric’s surface. Mater. Sci. 2013, 19, 169–173. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, H.; Zhou, H.; Lin, T. Self-cleaning, superhydrophobic cotton fabrics with excellent washing durability, solvent resistance and chemical stability prepared from an SU-8 derived surface coating. RSC Adv. 2015, 5, 61044–61050. [Google Scholar] [CrossRef]
- Deng, B.; Cai, R.; Yu, Y.; Jiang, H.; Wang, C.; Li, J.; Li, L.; Yu, M.; Li, J.; Xie, L.; et al. Laundering durability of superhydrophobic cotton fabric. Adv. Mater. 2010, 22, 5473–5477. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zheng, C.; Ye, W.; Huan, B. A facile dip-coating approach to prepare SiO2/fluoropolymer coating for superhydrophobic and superoleophobic fabrics with self-cleaning property. J. Appl. Polym. Sci. 2015, 132, 41458. [Google Scholar] [CrossRef]
- Huang, W.; Xing, Y.; Yu, B.; Shang, S.; Dai, J. Enhanced washing durability of hydrophobic coating on cellulose fabric using polycarboxylic acids. Appl. Surf. Sci. 2011, 257, 4443–4448. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Ding, J.; Feng, L.; Wang, X.; Lin, T. Durable, self-healing superhydrophobic and superoleophobic surfaces from fluorinated-decyl polyhedral oligomeric silsesquioxane and hydrolyzed fluorinated alkyl silane. Angew. Chem. Int. Ed. 2011, 50, 11433–11436. [Google Scholar] [CrossRef] [PubMed]
- Jassal, M.; Khungar, A.; Bajaj, P.; Sinha, T.J.M. Waterproof breathable polymeric coatings based on polyurethanes. J. Ind. Text. 2004, 33, 269–280. [Google Scholar] [CrossRef]
- Havenith, G.; Heus, R.; Lotens, W.A. Clothing ventilation, vapour resistance and permeability index: Changes due to posture, movement and wind. Ergonomics 1990, 33, 989–1005. [Google Scholar] [CrossRef]
- Majumdar, A.; Mukhopadhyay, S.; Yadav, R. Thermal properties of knitted fabrics made from cotton and regenerated bamboo cellulosic fibres. Int. J. Therm. Sci. 2010, 49, 2042–2048. [Google Scholar] [CrossRef]
- Shaid, A.; Furgusson, M.; Wang, L. Thermophysiological comfort analysis of aerogel nanoparticle incorporated fabric for fire fighter’s protective clothing. Chem. Mater. Eng. 2014, 2, 37–43. [Google Scholar]
- Kar, F.; Fan, J.; Yu, W. Comparison of different test methods for the measurement of fabric or garment moisture transfer properties. Meas. Sci. Technol. 2007, 18, 2033. [Google Scholar] [CrossRef]











| Samples | Functional groups | Wavenumber (cm−1) | Attributed to |
|---|---|---|---|
| Polycotton | O–H | 3382 (ѵ) | Stretching of hydroxyl group |
| CH2 | 2970 (ѵs) | Stretching of methyl group | |
| CH | 1425 (δ) | Bending of methyl group | |
| C–O–C | 1315 (a) | Asymmetric stretching of ester group | |
| C–O | 1017 (a) | Asymmetric stretching of carbon monoxide group | |
| TPU | NH | 3335 (ѵ) | Stretching of amide group |
| CH2 | 2955 (ѵa) | Symmetric stretching of methyl group | |
| C=O | 1728 | Stretching of carbonyl group | |
| C=C Benzene ring | 1597–1414 (δs) | Bending of symmetric of carbon with double bonding in ring | |
| PDMS-TMS | CH2 | 2975 (a) | Asymmetric stretching of methyl group |
| Si–CH3 | 1270–1250 (ѵa) | Asymmetric stretching of methyl and silicone group | |
| Si–(CH3)n | 870–700 (δ) | Bending stretching of silicone and no methyl groups | |
| Si–O–Si | 1059–1020 (ѵa) | Asymmetric stretching of silicones and oxygen group | |
| TPU-PDMS-TMS | NHCOO–Si–O–CH3 | 1713–749 | Crosslinking between TPU chain and PDMS-TMS groups |
| Chemicals | Surface tension (dynes/cm) | Polycotton | TPU | 8% PT | 2% TPT | 4% TPT | 6% TPT | 8% TPT |
|---|---|---|---|---|---|---|---|---|
| Triethylamine | 19.70 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tetrahydrofuran | 26.40 | 0 | 62 | 0 | 82 | 91 | 141 | 167 |
| n-Hexane | 18.43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dichloromethane | 26.80 | 0 | 70 | 5 | 0 | 16 | 30 | 35 |
| Acetone | 23.20 | 0 | 70 | 10 | 5 | 16 | 30 | 57 |
| Acetonitrile | 28.70 | 0 | 186 | 0 | 114 | 228 | 289 | 297 |
| Methanol | 22.10 | 0 | 146 | 0 | 69 | 75 | 85 | 137 |
| Toluene | 28.40 | 0 | 185 | 0 | 0 | 0 | 0 | 0 |
| Dimethylformamide | 36.70 | 0 | 300 | 300 | 300 | 300 | 300 | 300 |
| Acetic acids | 27.00 | 0 | 300 | 300 | 300 | 300 | 300 | 300 |
| Sodium hydroxide | 101.04 | 0 | 300 | 300 | 300 | 300 | 300 | 300 |
| Butadiene | 47.01 | 0 | 300 | 300 | 300 | 31 | 16 | 10 |
| Paraffin oil | 26.00 | 0 | 300 | 16 | 300 | 45 | 50 | 57 |
| n-Hexadecane | 25.30 | 0 | 300 | 300 | 118 | 30 | 29 | 28 |
| n-Decane | 23.80 | 0 | 40 | 0 | 13 | 6 | 5 | 4 |
| n-heptane | 20.10 | 0 | 300 | 0 | 300 | 300 | 300 | 300 |
| iso-propyl alcohol | 23.00 | 10 | 300 | 10 | 300 | 300 | 300 | 10 |
| Sulphuric acids | 84.00 | 0 | 300 | 300 | 300 | 300 | 300 | 0 |
| MMT index | Polycotton | TPU | 8% PT | 2–8% TPT | ||||
|---|---|---|---|---|---|---|---|---|
| Top surface | Bottom surface | Top surface | Bottom surface | Top surface | Bottom surface | Top surface | Bottom surface | |
| Wetting time (s) | 5.90 | 5.99 | 4.68 | 12.23 | 8.42 | 120.00 | 7.58–9.54 | 120.00 |
| Absorption rate (&/s) | 5.73 | 15.93 | 67.98 | 4.89 | 253.66 | 0.00 | 90.20–406.33 | 0.00 |
| Maximum wetted radius (mm) | 15.00 | 15.00 | 25.00 | 0.00 | 5.00 | 0.00 | 5.00 | 0.00 |
| Spreading speed (mm/s) | 2.47 | 3.20 | 4.84 | 0.00 | 0.58 | 0.00 | 0.51–0.64 | 0.00 |
| One way transport capability | 62.08 | −624.90 | −995.77 | −988.00–−1086.26 | ||||
| OMMC | 0.47 | 0.04 | 0 | 0 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moiz, A.; Padhye, R.; Wang, X. Coating of TPU-PDMS-TMS on Polycotton Fabrics for Versatile Protection. Polymers 2017, 9, 660. https://doi.org/10.3390/polym9120660
Moiz A, Padhye R, Wang X. Coating of TPU-PDMS-TMS on Polycotton Fabrics for Versatile Protection. Polymers. 2017; 9(12):660. https://doi.org/10.3390/polym9120660
Chicago/Turabian StyleMoiz, Arsheen, Rajiv Padhye, and Xin Wang. 2017. "Coating of TPU-PDMS-TMS on Polycotton Fabrics for Versatile Protection" Polymers 9, no. 12: 660. https://doi.org/10.3390/polym9120660
APA StyleMoiz, A., Padhye, R., & Wang, X. (2017). Coating of TPU-PDMS-TMS on Polycotton Fabrics for Versatile Protection. Polymers, 9(12), 660. https://doi.org/10.3390/polym9120660