The Preparations and Water Vapor Barrier Properties of Polyimide Films Containing Amide Moieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(Amic Acid) Copolymers and Preparations of PI Films
2.3. Methods
3. Results and Discussion
3.1. Synthesis and Characteristics of the Polyimide Films
3.2. Aggregation Structures and Density of the Polyimide Films
3.3. Surface Hydrophilicity and Water Absorption Properties of the Polyimide Films
3.4. Mechanical Properties of the Polyimide Films
3.5. Water Vapor Barrier Properties of the Polyimide Films
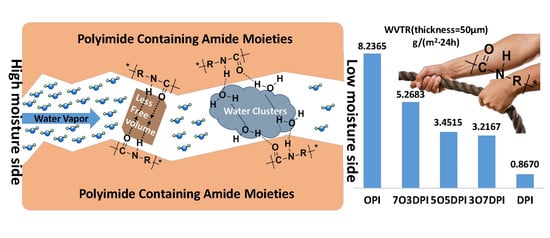
3.6. Mechanism of Water Vapor Barrier Properties of the Polyimides
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harper, C.A. Electronic Materials and Processes Handbook, 3rd ed.; McGraw-Hi: New York, NY, USA, 2005; ISBN 9780071402149. [Google Scholar]
- Milek, J.T. Polyimide Plastic Technology: A State-of-the-Art Report; Electronic Properties Information Centre, Hughes Aircraft: Culver City, CA, USA, 1965. [Google Scholar]
- Saba, N.; Tahir, P.M.; Jawaid, M. A Review on potentiality of nano filler/natural fiber filled polymer hybrid composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Mittal, K.L. Polyimides: Fundementals and Applications; Mercel Dekker: New York, NY, USA, 1996; ISBN 0824794664. [Google Scholar]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic Bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Wang, W.Y.; Hui, P.; Wat, E. Enhanced transdermal permeability via constructing the porous structure of poloxamer-based hydrogel. Polymers 2016, 8, 406. [Google Scholar] [CrossRef]
- Yucel, O.; Unsal, E.; Harvey, J. Enhanced gas barrier and mechanical properties in organoclay reinforced multi-layer poly(amide-imide) nanocomposite film. Polymer 2014, 55, 4091–4101. [Google Scholar] [CrossRef]
- Zhou, L.Z. Microelectronic Device Package-Packaging Materials and Packaging Technology; Chemical Industry Press: Beijing, China, 2006; pp. 7–10. ISBN 9787502590376. [Google Scholar]
- Hocker, S.; Smith, N.H.; Schniepp, H.C. Enhancing polyimide’s water barrier properties through addition of functionalized graphene oxide. Polymer 2016, 93, 23–29. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhang, G.P.; Sun, R. Research progress of encapsulation materials for OLED. J. Integr. Technol. 2014, 3, 92–101. [Google Scholar]
- Schmid, M.; Saengerlaub, S.; Miesbauer, O. Water repellence and oxygen and water vapor barrier of PVOH-coated substrates before and after surface esterification. Polymers 2014, 6, 2764–2783. [Google Scholar] [CrossRef]
- Ghoshal, S.; Denner, P. Study of the Formation of poly(vinyl alcohol) Films. Macromolecules 2012, 45, 1913–1923. [Google Scholar] [CrossRef]
- Fotie, G.; Rampazzo, R.; Ortenzi, M.A. The effect of moisture on cellulose nanocrystals intended as a high gas barrier coating on flexible packaging materials. Polymers 2017, 9, 415. [Google Scholar] [CrossRef]
- Zhu, T.J. Measurement of the Permeability of Water Vapor through OLED Encapsulation. Master’s Thesis, University of Electronic Science and Technology of China, Chengdu, China, 2006. [Google Scholar]
- Huang, H.Y.; Huang, T.C.; Yeh, T.C. Advanced anticorrosive materials prepared from amine-capped aniline trimer-based electroactive polyimide-clay nanocomposite materials with synergistic effects of redox catalytic capability and gas barrier properties. Polymer 2011, 52, 2391–2400. [Google Scholar] [CrossRef]
- Wang, Y.Z. Vacuum Technology; Beihang University Press: Beijing, China, 2007; ISBN 9787810779548. [Google Scholar]
- Gómez, M.; Palza, H.; Quijada, R. Influence of organically-modified montmorillonite and synthesized layered silica nanoparticles on the properties of polypropylene and polyamide-6 nanocomposites. Polymers 2016, 8, 386. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Strand, S.; Vårum, K. Chitosan: Gels and interfacial properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Liang, Q.; Zhou, H.P.; Fu, R.L. Themal conductivity of AlN ceramicssintered with CaF2 and YF3. CtronSystSector 2004, 2, 648–653. [Google Scholar]
- Vaed, K.; Florkey, J.; Akbar, S.A. An additive micro-molding approach for the development of micro-machined ceramic substrates for RF applications. J. Microelectron. Mech. Syst. 2004, 30, 514–525. [Google Scholar] [CrossRef]
- Bandera, D.; Meyer, V.R.; Prevost, D. Polylactide/montmorillonite hybrid latex as a barrier coating for paper applications. Polymers 2016, 8, 75. [Google Scholar] [CrossRef]
- Chee, W.K.; Lim, H.N.; Huang, N.M. Nanocomposites of graphene/polymers: A review. RSC Adv. 2015, 5, 68014–68051. [Google Scholar] [CrossRef]
- Aldana, D.S.; Villa, E.D.; De Dios Hernández, M.; Sánchez, G.G.; Cruz, Q.R.; Gallardo, S.F.; Castillo, H.P.; Casarrubias, L.B. Barrier properties of polylactic acid in cellulose based packages using montmorillonite as filler. Polymers 2014, 6, 2386–2403. [Google Scholar] [CrossRef]
- Gaska, K.; Kádár, R.; Rybak, A. Gas Barrier, Thermal, mechanical and rheological properties of highly aligned graphene-LDPE nanocomposites. Polymers 2017, 9, 294. [Google Scholar] [CrossRef]
- Li, X.L.; Ma, H.A.; Zuo, G.H. Low temperature sintering of high density aluminium nitride ceramics without additives at high pressure. Scipta Mater. 2007, 56, 1015–1018. [Google Scholar] [CrossRef]
- Ge, J.J.; Li, C.Y.; Xue, G.; Mann, I.K.; Zhang, D.; Harris, F.W.; Cheng, S.Z.D.; Hong, S.C.; Zhuang, X.; Shen, Y.R. Rubbing-Induced Molecular Reorientation on an Alignment Surface of an Aromatic Polyimide Containing Cyanobiphenyl Side Chains. J. Am. Chem. Soc. 2001, 123, 5768–5776. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Cho, W.J.; Ha, C.S.; Ando, S.; Kim, Y.K.; Park, C.H.; Lee, K. Flexible organic electroluminescent devices based on fluorine-containing volorless polyimide substrates. Adv. Mater. 2002, 14, 1275–1279. [Google Scholar] [CrossRef]
- Liu, Y.W.; Qian, C.; Qu, L.J.; Wu, Y.N.; Zhang, Y.; Wu, X.H.; Zou, B.; Chen, W.X.; Chen, Z.Q.; Chi, Z.G.; et al. A bulk dielectric polymer film with intrinsic ultralow dielectric constant and outstanding comprehensive properties. Chem. Mater. 2015, 27, 6543–6549. [Google Scholar] [CrossRef]
- Buntinx, M.; Willems, G.; Knockaert, G. Evaluation of the thickness and oxygen transmission rate before and after thermoforming mono- and multi-layer sheets into trays with variable depth. Polymers 2014, 6, 3019–3043. [Google Scholar] [CrossRef]
- Larson, S.E.; Slaby, J. Comparison of various substrate technologies under steady state and transient conditions. In Proceedings of the Thermal and Thermomechanical Phenomena in Electronic Systems, Las Vegas, NV, USA, 1–4 June 2004. [Google Scholar] [CrossRef]
- Liu, Q.J.; Li, X.Y.; Chen, G.W. Research and application progress on barrier polymer composites. Plast. Sci. Technol. 2013, 41, 104–108. [Google Scholar]
- Sehaqui, H.; Kochumalayil, J.; Liu, A. Multifunctional nanoclay hybrids of high toughness, thermal, and barrier performances. ACS Appl. Mater. Interface 2013, 5, 7613–7620. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.S.; Zhang, Z.C.; Shi, T.J. Application of biodegradable polyhydroxybutyrate in tissue engineering. J. Funct. Polym. 2001, 3, 361–364. [Google Scholar]
- Li, S.L. Measurement of Gas Permeability of OLED Packaging Materials. Master’s Thesis, University of Electronic Science and Technology of China, Chengdu, China, 2009. [Google Scholar]
- Huang, W.D. Research on Moisture-Proof Film Technology in High Reliability Electronic Package. Ph.D. Thesis, Shanghai Institute of Microsystem and Information Technology, Shanghai, China, 2003. [Google Scholar]
- Park, J.S.; Chae, H.; Chung, H.K. Thin film encapsulation for flexible AM-OLED: A review. Semicond. Sci. Technol. 2011, 26, 034001. [Google Scholar] [CrossRef]
- Massey, L.K. Permeability Properties of Plastics and Elastomers: A Guide to Packaging and Barrier Materials/Plastics Design Library; William Andrew: New York, NY, USA, 2003; ISBN 1884207979. [Google Scholar]
- Ping, Z.H.; Nguyen, Q.T.; Chen, S.M. States of water in different hydrophilic polymers-DSC and FTIR studies. Polymer 2001, 42, 8461–8467. [Google Scholar] [CrossRef]
- Yucel, O.; Unsal, E.; Cakmak, M. Temporal evolution of optical gradients during drying in cast polymer solutions. Macromolecules 2013, 46, 7112–7117. [Google Scholar] [CrossRef]
- Unsal, E.; Drum, J.; Yucel, O. Real-time measurement system for tracking birefringence, weight, thickness, and surface temperature during drying of solution cast coatings and films. Rev. Sci. Instrum. 2012, 83, 352. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chen, F.; Hedenqvist, M.S. Structural Changes of Gluten/Glycerol Plastics under Dry and Moist Conditions and during Tensile Tests. ACS Sustain. Chem. Eng. 2016, 4, 3388–3397. [Google Scholar] [CrossRef]
- Hyoe, H.; Tatsuko, H. Interaction between water and hydrophilic polymers. Thermochim. Acta 1998, 308, 3–22. [Google Scholar] [CrossRef]
- Nelson, R. The determination of moisture transitions in cellulosic materials using differential scanning calorimetry. J. Appl. Polym. Sci. 1977, 21, 645–654. [Google Scholar] [CrossRef]
- Ye, Y.S.; Rick, J.; Hwang, B.J. Water soluble polymers as proton exchange membranes for fuel cells. Polymers 2012, 4, 913–963. [Google Scholar] [CrossRef]
- Kadajji, V.G.; Betageri, G.V. Water soluble polymers for pharmaceutical applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef]
- Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Studies on bound water of celluslose by different scanning calorimetry. Text. Res. J. 1981, 51, 607. [Google Scholar] [CrossRef]
- Magne, E.C.; Portas, H.J.; Wakeham, H.A. Design and analysis of the homogeneous and heterogeneous distribution of water confined within colloidal polymer particles. Colloid Polym. Sci. 2013, 291, 143–156. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T.M. Phase transition temperature of water restrained in polysulfone hollow fibres. J. Therm. Anal. 1993, 40, 727–733. [Google Scholar] [CrossRef]
- Miltner, H.E.; Assche, G.V.; Pozsgay, A. Restricted chain segment mobility in poly(amide) 6/clay nanocomposites evidenced by quasi-isothermal crystallization. Polymer 2006, 47, 826–835. [Google Scholar] [CrossRef]











| Company | Model | Type | WVTR (50 μm)/g·(m2·24 h)−1 |
|---|---|---|---|
| uPont | Kapton | Polyimide(PI) Film | 27 |
| UBE | Upilex R | PI Film | 11.2 |
| Sabic Innovative Plastics | Ultem 1000 | Polyetherimide (PEI) Film | 60 |
| Dow Chemical | Trycite Oriented | Polystyrene(PS) Film | 70 |
| Teijin | Mylar | Polyethylene terephthalate(PET) Film | 10.6 |
| EMS Chemie | Grivory G21 | Nylon | 7 |
| Chevron Phillips | Ryton | Polyphenylene sulfide(PPS) Films | 6 |
| Sample | M1/g | M2/g | Water absorption/% | Water contact angle/° |
|---|---|---|---|---|
| OPI | 0.6001 | 0.6049 | 0.7998 | 79.4 |
| 7O3DPI | 0.3065 | 0.3112 | 1.5334 | 75.3 |
| 5O5DPI | 0.2563 | 0.2618 | 2.1459 | 72.7 |
| 3O7DPI | 0.3072 | 0.3154 | 2.6693 | 68.1 |
| DPI | 0.2505 | 0.2596 | 3.6327 | 64.7 |
| Sample | Tensile strength/MPa | Elongation at break/% | Elastic modulus/GPa |
|---|---|---|---|
| OPI | 136.8 ± 4.7 | 14.4 ± 2.3 | 3.20 ± 0.51 |
| 7O3DPI | 140.2 ± 4.5 | 12.6 ± 1.3 | 3.58 ± 0.38 |
| 5O5DPI | 152.7 ± 4.3 | 12.5 ± 1.1 | 3.65 ± 0.49 |
| 3O7DPI | 165.4 ± 6.4 | 11.0 ± 2.4 | 3.66 ± 0.30 |
| DPI | 174.3 ± 0.6 | 9.8 ± 1.6 | 4.08 ± 0.29 |
| Sample | Tensile strength/MPa | Elongation at break/% | Elastic modulus/GPa |
|---|---|---|---|
| OPI-water | 134.6 ± 2.5 | 13.5 ± 2.1 | 3.07 ± 0.39 |
| 5O5DPI-water | 155.9 ± 3.9 | 11.7 ± 1.9 | 3.70 ± 0.37 |
| DPI-water | 176.0 ± 3.5 | 9.6 ± 1.4 | 4.27 ± 0.37 |
| Sample | Transmission coefficient/g·cm·(cm2·s·Pa)−1 | WVTR/g·(m2·24 h)−1 |
|---|---|---|
| OPI | 1.261 × 10−12 | 8.2365 |
| 7O3DPI | 8.063 × 10−13 | 5.2683 |
| 5O5DPI | 5.282 × 10−13 | 3.4515 |
| 3O7DPI | 4.923 × 10−13 | 3.2167 |
| DPI | 1.327 × 10−13 | 0.8670 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Yu, Q.; Zhu, L.; Liu, S.; Chi, Z.; Chen, X.; Zhang, Y.; Xu, J. The Preparations and Water Vapor Barrier Properties of Polyimide Films Containing Amide Moieties. Polymers 2017, 9, 677. https://doi.org/10.3390/polym9120677
Zhang K, Yu Q, Zhu L, Liu S, Chi Z, Chen X, Zhang Y, Xu J. The Preparations and Water Vapor Barrier Properties of Polyimide Films Containing Amide Moieties. Polymers. 2017; 9(12):677. https://doi.org/10.3390/polym9120677
Chicago/Turabian StyleZhang, Kai, Qiaoxi Yu, Longji Zhu, Siwei Liu, Zhenguo Chi, Xudong Chen, Yi Zhang, and Jiarui Xu. 2017. "The Preparations and Water Vapor Barrier Properties of Polyimide Films Containing Amide Moieties" Polymers 9, no. 12: 677. https://doi.org/10.3390/polym9120677
APA StyleZhang, K., Yu, Q., Zhu, L., Liu, S., Chi, Z., Chen, X., Zhang, Y., & Xu, J. (2017). The Preparations and Water Vapor Barrier Properties of Polyimide Films Containing Amide Moieties. Polymers, 9(12), 677. https://doi.org/10.3390/polym9120677