Preparation of Fouling-Resistant Nanofibrous Composite Membranes for Separation of Oily Wastewater
Abstract
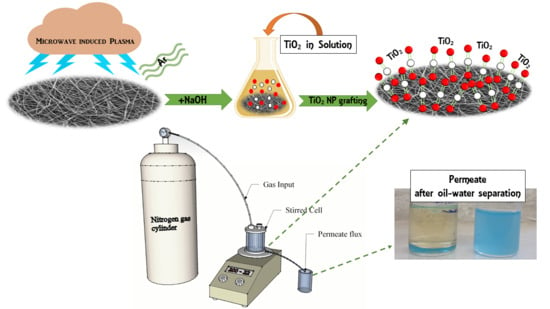
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Nanofiber Web
2.2. Formation of a Nanofibrous Composite Membrane
2.3. Surface Treatment
- Solution A: 5 wt % of titanium isopropoxide (Sigma-Aldrich, Sigma-Aldrich Sp. Z.o.o., Poznan, Poland) was mixed in a propanol solvent at 50 °C.
- Solution B: 5 wt % of diluted acetic acid was prepared.
- Solution C: Solution B was slowly poured into solution A at a ratio of 50:50 v/v.
- Solution D: Solution C was heated to remove any water.
- Solution E: Sodium hydroxide (NaOH) was used to neutralize the pH of solution D.
2.4. Filtration and Self-Cleaning Experiments
3. Results
3.1. Membrane Characterization
3.2. Filtration and Self-Cleaning Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, M.; Gao, Y.; Guo, X.; Shi, Z.; Chen, J.; Shi, F. A functionally integrated device for effective and facile oil spill cleanup. Langmuir 2011, 27, 7371–7375. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.L.; Sumathi, S.; Hameed, B.H. Coagulation of residue oil and suspended solid in palm oil mill effluent by chitosan, alum and PAC. Chem. Eng. J. 2006, 118, 99–105. [Google Scholar] [CrossRef]
- Paul, U.C.; Fragouli, D.; Bayer, I.S.; Athanassiou, A. Functionalized cellulose networks for efficient oil removal from oil–water emulsions. Polymers 2016, 8, 52. [Google Scholar] [CrossRef]
- Liu, T.; Chen, S.; Liu, H. Oil adsorption and reuse performance of multi-walled carbon nanotubes. Procedia Eng. 2015, 102, 1896–1902. [Google Scholar] [CrossRef]
- Okiel, K.; El-Sayed, M.; El-Kady, M.Y. Treatment of oil–water emulsions by adsorption onto activated carbon, bentonite and deposited carbon. Egypt. J. Pet. 2011, 20, 9–15. [Google Scholar] [CrossRef]
- Al-Shamrani, A.A.; James, A.; Xiao, H. Destabilisation of oil–water emulsions and separation by dissolved air flotation. Water Res. 2002, 36, 1503–1512. [Google Scholar] [CrossRef]
- Felizardo, P.; Joana Neiva Correia, M.; Raposo, I.; Mendes, J.F.; Berkemeier, R.; Bordado, J.M. Production of biodiesel from waste frying oils. Waste Manag. 2006, 26, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Li, J.; Zhou, W.; Zhang, R.; Ma, G.; Su, Z. Improved stability of emulsions in preparation of uniform small-sized konjac glucomanna (KGM) microspheres with epoxy-based polymer membrane by premix membrane emulsification. Polymers 2016, 8, 53. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, W.; Zhang, F.; Xing, T.; Jin, J. Superhydrophilic in-situ-cross-linked zwitterionic polyelectrolyte/PVDF-blend membrane for highly efficient oil/water emulsion separation. ACS Appl. Mater. Interfaces 2017, 9, 9603–9613. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Hao, T.; Wang, P.; Zhang, Y.; Cheng, B.; Yuan, T.; Meng, J. Surface modified glass fiber membranes with superior chemical and thermal resistance for O/W separation. Chem. Eng. J. 2017, 309, 30–40. [Google Scholar] [CrossRef]
- Cumming, I.W.; Holdich, R.G.; Smith, I.D. The rejection of oil using an asymmetric metal microfilter to separate an oil in water dispersion. Water Res. 1999, 33, 3587–3594. [Google Scholar] [CrossRef]
- Koehler, J.A.; Ulbricht, M.; Belfort, G. Intermolecular forces between proteins and polymer films with relevance to filtration. Langmuir 1997, 13, 4162–4171. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Antifouling and antimicrobial polymer membranes based on bioinspired polydopamine and strong hydrogen-bonded poly(N-vinyl pyrrolidone). ACS Appl. Mater. Interfaces 2013, 5, 12895–12904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jiang, J.; Zhang, Q.; Gao, F.; Zhan, X.; Chen, F. Ultralow oil-Fouling heterogeneous poly(ether sulfone) ultrafiltration membrane via blending with novel amphiphilic fluorinated gradient copolymers. Langmuir 2016, 32, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Xue, A.; Zhao, Y.; Li, M.; Wang, H.; Xing, W. Grafting polyacrylic acid brushes onto zirconia membranes: Fouling reduction and easy-cleaning properties. Sep. Purif. Technol. 2013, 114, 53–63. [Google Scholar] [CrossRef]
- Zhao, X.; Su, Y.; Chen, W.; Peng, J.; Jiang, Z. Grafting perfluoroalkyl groups onto polyacrylonitrile membrane surface for improved fouling release property. J. Membr. Sci. 2012, 415–416, 824–834. [Google Scholar] [CrossRef]
- Ju, H.; McCloskey, B.D.; Sagle, A.C.; Wu, Y.H.; Kusuma, V.A.; Freeman, B.D. Crosslinked poly(ethylene oxide) fouling resistant coating materials for oil/water separation. J. Membr. Sci. 2008, 307, 260–267. [Google Scholar] [CrossRef]
- Jing, B.; Wang, H.; Lin, K.Y.; McGinn, P.; Na, C.; Zhu, Y. A facile method to functionalize engineering solid membrane supports for rapid and efficient oil–water separation. Polymer 2013, 54, 5771–5778. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, J.; Zhu, D.; Zheng, J.; Handschuh-Wang, S.; Zhou, X.; Zhang, J.; Liu, Y.; Liu, Z.; He, C.; et al. Hydrophilic sponges for leaf-inspired continuous pumping of liquids. Adv. Sci. 2017, 4, 1700028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, Q.; Pan, X.; Jin, Y.; Lu, W.; Ding, D.; Guo, Q. Graphene oxide/polyacrylonitrile fiber hierarchical-structured membrane for ultra-fast microfiltration of oil-water emulsion. Chem. Eng. J. 2017, 307, 643–649. [Google Scholar] [CrossRef]
- Schulze, A.; Breite, D.; Kim, Y.; Schmidt, M.; Thomas, I.; Went, M.; Fischer, K.; Prager, A. bio-inspired polymer membrane surface cleaning. Polymers 2017, 9, 97. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Xu, X.; Zhu, X.; Men, X.; Zhou, X. Superhydrophilic–superoleophobic coatings. J. Mater. Chem. 2012, 22, 2834–2837. [Google Scholar] [CrossRef]
- Wei, X.; Fei, Y.; Shi, Y.; Chen, J.; Lv, B.; Chen, Y.; Xiang, H. Hemocompatibility and ultrafiltration performance of PAN membranes surface-modified by hyperbranched polyesters. Polym. Adv. Technol. 2016, 27, 1569–1576. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, C.; Wang, X.; Liu, Y.; Hu, H.; Guo, Y.; Ma, K.; Fei, B.; Xin, J. Beads-on-string structured nanofibers for smart and reversible oil/water separation with outstanding antifouling property. ACS Appl. Mater. Interfaces 2016, 8, 25612–25620. [Google Scholar] [CrossRef] [PubMed]
- Daoud, W.A.; Leung, S.K.; Tung, W.S.; Xin, J.H.; Cheuk, K.; Qi, K. Self-cleaning keratins. Chem. Mater. 2008, 20, 1242–1244. [Google Scholar] [CrossRef]
- Kaihong, Q.; Wang, X.; Xin, J.H. Photocatalytic self-cleaning textiles based on nanocrystalline titanium dioxide. Text. Res. J. 2011, 81, 101–110. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Nanocomposite electrospun nanofiber membranes for environmental remediation. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, F. Mechanically enhanced electrospun nanofibers for wastewater treatment. In Proceedings of the International Conference on Advances in Energy Systems and Environmental Engineering, Wroclaw, Poland, 2–5 July 2017; Volume 7. [Google Scholar]
- Yalcinkaya, F. Preparation of various nanofiber layers using wire electrospinning system. Arab. J. Chem. 2016, 12. [Google Scholar] [CrossRef]
- Jiříček, T.; Komárek, M.; Chaloupek, J.; Lederer, T. Flux enhancement in membrane distillation using nanofiber membranes. J. Nanomater. 2016, 2016, 9327431. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, D.; Lin, S. Composite membrane with underwater-oleophobic surface for anti-oil-fouling membrane distillation. Environ. Sci. Technol. 2016, 50, 3866–3874. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, R.; Tian, M.; Qiu, C.; Fane, A.G. Fabrication of polyvinylidene fluoride (PVDF) nanofiber membranes by electro-spinning for direct contact membrane distillation. J. Membr. Sci. 2013, 425–426, 30–39. [Google Scholar] [CrossRef]
- Mei, Y.; Yao, C.; Fan, K.; Li, X. Surface modification of polyacrylonitrile nanofibrous membranes with superior antibacterial and easy-cleaning properties through hydrophilic flexible spacers. J. Membr. Sci. 2012, 417–418, 20–27. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Yalcinkaya, B.; Pazourek, A.; Mullerova, J.; Stuchlik, M.; Maryska, J. Surface modification of electrospun PVDF/PAN nanofibrous layers by low vacuum plasma treatment. Int. J. Polym. Sci. 2016, 2016. [Google Scholar] [CrossRef]
- Kang, Y.H.; Ahn, K.; Jeong, S.Y.; Bae, J.S.; Jin, J.S.; Kim, H.G.; Hong, S.W.; Cho, C.R. Effect of plasma treatment on surface chemical-bonding states and electrical properties of polyacrylonitrile nanofibers. Thin Solid Films 2011, 519, 7090–7094. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Siekierka, A.; Bryjak, M.; Maryska, J. Preparation of various nanofibrous composite membranes using wire electrospinning for oil-water separation. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2017; Volume 254, p. 102011. [Google Scholar]
- Yalcinkaya, B.; Yalcinkaya, F.; Chaloupek, J. Thin film nanofibrous composite membrane for dead-end seawater desalination. J. Nanomater. 2016, 2016, 2694373. [Google Scholar] [CrossRef]
- Gancarz, I.; Bryjak, M.; Wolska, J.; Siekierka, A.; Kujawski, W. Membranes with a plasma deposited titanium isopropoxide layer. Chem. Pap. 2016, 70. [Google Scholar] [CrossRef]
- Siekierka, A.; Kujawa, J.; Kujawski, W.; Bryjak, M. Lithium dedicated adsorbent for the preparation of electrodes useful in the ion pumping method. Sep. Purif. Technol. 2018, 194, 231–238. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Robert, D.; Weber, J.V. Preparation of TiO2 nanoparticles by Sol-Gel route. Int. J. Photoenergy 2003, 5, 153–158. [Google Scholar] [CrossRef]
- Uosaki, K.; Yano, T.; Nihonyanagi, S. Interfacial water structure at as-prepared and UV-induced hydrophilic TiO2 surfaces studied by sum frequency generation spectroscopy and quartz crystal microbalance. J. Phys. Chem. B 2004, 108, 19086–19088. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Yalcinkaya, B.; Hruza, J.; Hrabak, P. Effect of nanofibrous membrane structures on the treatment of wastewater microfiltration. Sci. Adv. Mater. 2017, 9, 747–757. [Google Scholar] [CrossRef]
- Na, L.; Liu, Z.; Xu, S. Dynamically formed poly(vinyl alcohol) ultrafiltration membranes with good anti-fouling characteristics. J. Membr. Sci. 2000, 169, 17–28. [Google Scholar] [CrossRef]
- Clouet, F.; Shi, M.K. Interactions of polymer model surfaces with cold plasmas: Hexatriacontane as a model molecule of high-density polyethylene and octadecyl octadecanoate as a model of polyester. I. Degradation rate versus time and power. J. Appl. Polym. Sci. 1992, 46, 1955–1966. [Google Scholar] [CrossRef]
- Li, J.H.; Xu, Y.Y.; Zhu, L.P.; Wang, J.H.; Du, C.H. Fabrication and characterization of a novel TiO2 nanoparticle self-assembly membrane with improved fouling resistance. J. Membr. Sci. 2009, 326, 659–666. [Google Scholar] [CrossRef]
- Bryjak, M.; Hodge, H.; Dach, B. Modification of porous polyacrylonitrile membrane. Die Angew. Makromol. Chem. 1998, 260, 25–29. [Google Scholar] [CrossRef]
- Yang, M.C.; Tong, J.H. Loose ultrafiltration of proteins using hydrolyzed polyacrylonitrile hollow fiber. J. Membr. Sci. 1997, 132, 63–71. [Google Scholar] [CrossRef]
- Abedi, M.; Sadeghi, M.; Pourafshari Chenar, M. Improving antifouling performance of PAN hollow fiber membrane using surface modification method. J. Taiwan Inst. Chem. Eng. 2015, 55, 42–48. [Google Scholar] [CrossRef]
- Grujic-Brojcin, M.; Scepanovic, M.; Dohcevic-Mitrovic, Z.; Popovic, Z.V. Infrared study of nonstoichiometric anatase TiO2 nanopowders. Sci. Sinter. 2006, 38, 183–189. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Kumar, P.M.; Badrinarayanan, S.; Sastry, M. Nanocrystalline TiO2 studied by optical, FTIR and X-ray photoelectron spectroscopy: Correlation to presence of surface states. Thin Solid Films 2000, 358, 122–130. [Google Scholar] [CrossRef]
- Pan, H.; Wang, X.; Xiao, S.; Yu, L.; Zhang, Z. Preparation and characterization of TiO2 nanoparticles surface-modified by octadecyltrimethoxysilane. Indian J. Eng. Mater. Sci. 2013, 20, 561–567. [Google Scholar]
- Chen, F.; Lu, Y.; Liu, X.; Song, J.; He, G.; Tiwari, M.K.; Carmalt, C.J.; Parkin, I.P. Table salt as a template to prepare reusable porous PVDF–MWCNT foam for separation of immiscible oils/organic solvents and corrosive aqueous solutions. Adv. Funct. Mater. 2017, 27, 1702926. [Google Scholar] [CrossRef]












| Abbreviation of the Sample | Plasma Modification | Chemical Modification | UV Irradiation |
|---|---|---|---|
| P0* | - | - | - |
| P1 | 5 min Plasma + 20 min exposed to atmosphere | Immersed into NaOH for 24 h | - |
| P2 | 5 min Plasma + 20 min exposed to atmosphere | Immersed into solution E for 2 days | - |
| P3 | 5 min Plasma + 20 min exposed to atmosphere | Immersed into NaOH for 24 h and solution E for 2 days | - |
| P4 | 5 min Plasma + 20 min exposed to atmosphere | Immersed into NaOH for 24 h and solution E for 2 days | 4 min under UV light |
| Polymer | Viscosity (Pa.s) | Basis Weight of Nanofiber (g/m2) | Fiber Diameter (nm) | Porosity (%) | Avr. Pore Size (nm) | Contact Angle (°) |
|---|---|---|---|---|---|---|
| PVDF/PAN | 0.35 | 0.76 ± 0.50 | 110.18 ± 19.90 | >85 | 820 ± 32 | 92.7 ± 3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalcinkaya, F.; Siekierka, A.; Bryjak, M. Preparation of Fouling-Resistant Nanofibrous Composite Membranes for Separation of Oily Wastewater. Polymers 2017, 9, 679. https://doi.org/10.3390/polym9120679
Yalcinkaya F, Siekierka A, Bryjak M. Preparation of Fouling-Resistant Nanofibrous Composite Membranes for Separation of Oily Wastewater. Polymers. 2017; 9(12):679. https://doi.org/10.3390/polym9120679
Chicago/Turabian StyleYalcinkaya, Fatma, Anna Siekierka, and Marek Bryjak. 2017. "Preparation of Fouling-Resistant Nanofibrous Composite Membranes for Separation of Oily Wastewater" Polymers 9, no. 12: 679. https://doi.org/10.3390/polym9120679
APA StyleYalcinkaya, F., Siekierka, A., & Bryjak, M. (2017). Preparation of Fouling-Resistant Nanofibrous Composite Membranes for Separation of Oily Wastewater. Polymers, 9(12), 679. https://doi.org/10.3390/polym9120679