Confinement Effects on Polymer Dynamics: Thermo-Responsive Behaviours of Hydroxypropyl Cellulose Polymers in Phospholipid-Coated Droplets (Water-in-Oil Emulsion)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HPC Solution Droplets
2.3. Microscopic Observation under Temperature Control
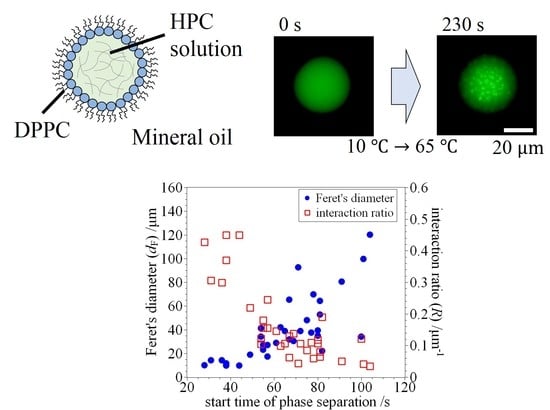
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HPC | hydroxypropyl cellulose |
| W/O droplets | water-in-oil droplets |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DPPG | 1,2-dipalmitoyl-sn-glycero-3-phospho-(1-rac-glycerol) |
References
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Fujii, Y.; Nishio, I. Deformation of Lipid Membranes Containing Photoresponsive Molecules in Response to Ultraviolet Light. J. Phys. Chem. B 2014, 118, 4115–4121. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Horii, K.; Fujii, Y.; Nishio, I. Real-Time Observation of Liposome Bursting Induced by Acetonitrile. ChemPhysChem 2014, 15, 2909–2912. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Takashima, A.; Nishio, I. Effect of Dibucaine Hydrochloride on Raft-Like Lipid Domains in Model Membrane Systems. Med. Chem. Commun. 2015, 6, 1444–1451. [Google Scholar] [CrossRef]
- Hamada, T.; Morita, M.; Kishimoto, Y.; Komatsu, Y.; Vestergaard, M.; Takagi, M. Biomimetic Microdroplet Membrane Interface: Detection of the Lateral Localization of Amyloid Beta Peptides. J. Phys. Chem. Lett. 2010, 1, 170–173. [Google Scholar] [CrossRef]
- Hamada, T.; Fujimoto, R.; Shimobayashi, S.F.; Ichikawa, M.; Takagi, M. Molecular behavior of DNA in a cell-sized compartment coated by lipids. Phys. Rev. E 2015, 91, 062717. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Minton, A.P. Cell biology: Join the crowd. Nature 2003, 425, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Nishio, I.; Sun, S.T.; Swislow, G.; Tanaka, T. First observation of the coil–globule transition in a single polymer chain. Nature 1979, 281, 208–209. [Google Scholar] [CrossRef]
- Tanaka, T.; Soda, K.; Wada, A. Dynamical aspects of helix-coil transitions in polypeptides. I. J. Chem. Phys. 1973, 58, 5707–5715. [Google Scholar] [CrossRef]
- Tanaka, T.; Wada, A.; Suzuki, M. Dynamical aspects of helix-coil transitions in biopolymers. II. J. Chem. Phys. 1973, 59, 3799–3810. [Google Scholar] [CrossRef]
- Bonduelle, C.; Makni, F.; Severac, L.; Piedra-Arroni, E.; Serpentini, C.L.; Lecommandoux, S.; Pratviel, G. Smart metallopoly(l-glutamic acid) polymers: Reversible helix-to-coil transition at neutral pH. RSC Adv. 2016, 6, 84694–84697. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Yamashita, Y.; atsu Mukai, S.; Annaka, M.; Tokita, M. Phase separation in binary polymer solution: Gelatin/Poly(ethylene glycol) system. J. Mol. Liq. 2014, 200, 2–6. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yanagisawa, M.; Tokita, M. Sol-gel transition and phase separation in ternary system of gelatin-water-poly(ethylene glycol) oligomer. J. Mol. Liq. 2014, 200, 47–51. [Google Scholar] [CrossRef]
- Kusumaatmaja, H.; Li, Y.; Dimova, R.; Lipowsky, R. Intrinsic Contact Angle of Aqueous Phases at Membranes and Vesicles. Phys. Rev. Lett. 2009, 103, 238103. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kusumaatmaja, H.; Lipowsky, R.; Dimova, R. Wetting-Induced Budding of Vesicles in Contact with Several Aqueous Phases. J. Phys. Chem. B 2012, 116, 1819–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimova, R.; Lipowsky, R. Lipid membranes in contact with aqueous phases of polymer solutions. Soft Matter 2012, 8, 6409–6415. [Google Scholar] [CrossRef]
- Dimova, R.; Lipowsky, R. Giant Vesicles Exposed to Aqueous Two-Phase Systems: Membrane Wetting, Budding Processes, and Spontaneous Tubulation. Adv. Mater. Interfaces 2017, 4, 1600451. [Google Scholar] [CrossRef]
- Keating, C.D. Aqueous phase separation as a possible route to compartmentalization of biological molecules. Acc. Chem. Res. 2012, 45, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Nigorikawa, S.; Sakaue, T.; Fujiwara, K.; Tokita, M. Multiple patterns of polymer gels in microspheres due to the interplay among phase separation, wetting, and gelation. Proc. Natl. Acad. Sci. USA 2014, 111, 15894–15899. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Yanagisawa, M.; Sato, Y.T.; Fujiwara, K.; Yoshikawa, K. Cell-sized confinement in microspheres accelerates the reaction of gene expression. Sci. Rep. 2012, 2, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2014. [Google Scholar]
- Fortin, S.; Charlet, G. Phase diagram of aqueous solutions of (hydroxypropyl)cellulose. Macromolecules 1989, 22, 2286–2292. [Google Scholar] [CrossRef]
- Winnik, F.M.; Tamai, N.; Yonezawa, J.; Nishimura, Y.; Yamazaki, I. Temperature-induced phase transition of pyrene-labeled (hydroxypropyl) cellulose in water: Picosecond fluorescence studies. J. Phys. Chem. 1992, 96, 1967–1972. [Google Scholar] [CrossRef]
- Guido, S. Phase behavior of aqueous solutions of hydroxypropyl cellulose. Macromolecules 1995, 28, 4530–4539. [Google Scholar]
- Kabra, B.G.; Gehrke, S.H.; Spontak, R.J. Microporous, Responsive Hydroxypropyl Cellulose Gels. 1. Synthesis and Microstructure. Macromolecules 1998, 31, 2166–2173. [Google Scholar] [CrossRef]
- Adrados, B.; Galaev, I.; Nilsson, K.; Mattiasson, B. Size exclusion behavior of hydroxypropylcellulose beads with temperature-dependent porosity. J. Chromatogr. A 2001, 930, 73–78. [Google Scholar] [CrossRef]
- Xia, X.; Tang, S.; Lu, X.; Hu, Z. Formation and Volume Phase Transition of Hydroxypropyl Cellulose Microgels in Salt Solution. Macromolecules 2003, 36, 3695–3698. [Google Scholar] [CrossRef]
- Hamada, T.; Kishimoto, Y.; Nagasaki, T.; Takagi, M. Lateral phase separation in tense membranes. Soft Matter 2011, 7, 9061–9068. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, C.; Fujiwara, K.; Morita, M.; Kawamata, I.; Kawagishi, Y.; Sakai, A.; Murayama, Y.; Nomura, S.M.; Murata, S.; Takinoue, M.; et al. DNA cytoskeleton for stabilizing artificial cells. Proc. Natl. Acad. Sci. USA 2017, 114, 7228–7233. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, H.; Nishimura, K.; Suzuki, H.; Matsuura, T.; Yomo, T. Coupling of the fusion and budding of giant phospholipid vesicles containing macromolecules. Proc. Natl. Acad. Sci. USA 2012, 109, 5942–5947. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Horii, K.; Saito, A.; Takashima, A.; Nishio, I. Confinement Effects on Polymer Dynamics: Thermo-Responsive Behaviours of Hydroxypropyl Cellulose Polymers in Phospholipid-Coated Droplets (Water-in-Oil Emulsion). Polymers 2017, 9, 680. https://doi.org/10.3390/polym9120680
Yoshida K, Horii K, Saito A, Takashima A, Nishio I. Confinement Effects on Polymer Dynamics: Thermo-Responsive Behaviours of Hydroxypropyl Cellulose Polymers in Phospholipid-Coated Droplets (Water-in-Oil Emulsion). Polymers. 2017; 9(12):680. https://doi.org/10.3390/polym9120680
Chicago/Turabian StyleYoshida, Kazunari, Keitaro Horii, Azusa Saito, Akito Takashima, and Izumi Nishio. 2017. "Confinement Effects on Polymer Dynamics: Thermo-Responsive Behaviours of Hydroxypropyl Cellulose Polymers in Phospholipid-Coated Droplets (Water-in-Oil Emulsion)" Polymers 9, no. 12: 680. https://doi.org/10.3390/polym9120680
APA StyleYoshida, K., Horii, K., Saito, A., Takashima, A., & Nishio, I. (2017). Confinement Effects on Polymer Dynamics: Thermo-Responsive Behaviours of Hydroxypropyl Cellulose Polymers in Phospholipid-Coated Droplets (Water-in-Oil Emulsion). Polymers, 9(12), 680. https://doi.org/10.3390/polym9120680