Influence of Ligand Backbone Structure and Connectivity on the Properties of Phosphine-Sulfonate Pd(II)/Ni(II) Catalysts
Abstract
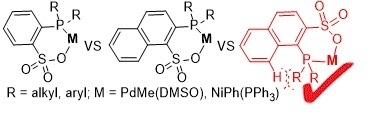
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guo, L.H.; Chen, C.L. (α-Diimine) palladium catalyzed ethylene polymerization and copolymerization with polar comonomers. Sci. China Chem. 2015, 58, 1663–1673. [Google Scholar] [CrossRef]
- Guo, L.H.; Dai, S.Y.; Sui, X.L.; Chen, C.L. Palladium and nickel catalyzed chain walking olefin polymerization and copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef]
- Guan, Z.; Cotts, P.M.; McCord, E.F.; McLain, S.J. Chain walking: A new strategy to control polymer topology. Science 1999, 283, 2059–2061. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Luo, S.; Jordan, R.F. Multiple Insertion of a Silyl Vinyl Ether by (α-Diimine)PdMe+ Species. J. Am. Chem. Soc. 2008, 130, 12892–12893. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jordan, R.F. Palladium-catalyzed dimerization of vinyl ethers to acetals. J. Am. Chem. Soc. 2010, 132, 10254–10255. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Luo, S.; Jordan, R.F. Cationic Polymerization and Insertion Chemistry in the Reactions of Vinyl Ethers with (α-Diimine)PdMe+ Species. J. Am. Chem. Soc. 2010, 132, 5273–5284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Fu, Z.S.; Pan, H.J.; Feng, W.; Chen, C.L.; Fan, Z.Q. Synthesis and application of binuclear α-diimine nickel/palladium catalysts with a conjugated backbone. Dalton Trans. 2014, 43, 2900–2906. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K.; Sui, X.L.; Pang, W.M.; Chen, C.L. Ethylene polymerization by xanthene-bridged dinuclear α-diimine NiII complexes. ChemCatChem 2016, 8, 434–440. [Google Scholar] [CrossRef]
- Na, Y.N.; Wang, X.; Lian, K.; Zhu, Y.; Li, W.; Luo, Y.; Chen, C.L. Dinuclear α–diimine Ni(II) and Pd(II) Catalyzed Ethylene Polymerization and Copolymerization. ChemCatChem 2017, 9, 1062–1066. [Google Scholar] [CrossRef]
- Wang, R.K.; Zhao, M.H.; Chen, C.L. Influence of ligand second coordination sphere effects on the olefin (co)polymerization properties of α-diimine Pd(II) catalysts. Polym. Chem. 2016, 7, 3933–3938. [Google Scholar] [CrossRef]
- Guo, L.H.; Dai, S.Y.; Chen, C.L. Investigations of the ligand electronic effects on α-diimine nickel(II) catalyzed ethylene polymerization. Polymers 2016, 8, 37. [Google Scholar] [CrossRef]
- Takano, S.; Takeuchi, D.; Osakada, K.; Akamatsu, N.; Shishido, A. Dipalladium Catalyst for Olefin Polymerization: Introduction of Acrylate Units into the Main Chain of Branched Polyethylene. Angew. Chem. Int. Ed. 2014, 53, 9246–9250. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.E.; Campos, J.; Daugulis, O.; Brookhart, M. Living Polymerization of Ethylene and Copolymerization of Ethylene/Methyl Acrylate Using “Sandwich” Diimine Palladium Catalysts. ACS Catal. 2015, 5, 456–464. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, W.J.; Daugulis, O.; Brookhart, M. Mechanistic Studies of Pd(II)-Catalyzed Copolymerization of Ethylene and Vinylalkoxysilanes: Evidence for a β-Silyl Elimination Chain Transfer Mechanism. J. Am. Chem. Soc. 2016, 138, 16120–16129. [Google Scholar]
- Younkin, T.R.; Connor, E.F.; Henderson, J.I.; Friedrich, S.K.; Grubbs, R.H.; Bansleben, D.A. Neutral, Single-Component Nickel(II) Polyolefin Catalysts That Tolerate Heteroatoms. Science 2000, 287, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Friedrich, S.; Younkin, T.R.; Li, R.T.; Grubbs, R.H.; Bansleben, D.A.; Day, M.W. Neutral Nickel(II)-Based Catalysts for Ethylene Polymerization. Organometallics 1998, 17, 3149–3151. [Google Scholar] [CrossRef]
- Weberski, M.P.; Chen, C.; Delferro, M.; Zuccaccia, C.; Macchioni, A.; Marks, T.J. Suppression of β-hydride chain transfer in nickel(II)-catalyzed ethylene polymerization via weak fluorocarbon ligand–product interactions. Organometallics 2012, 31, 3773–3789. [Google Scholar] [CrossRef]
- Weberski, M.P.; Chen, C.; Delferro, M.; Marks, T.J. Ligand Steric and Fluoroalkyl Substituent Effects on Enchainment Cooperativity and Stability in Bimetallic Nickel(II) Polymerization Catalysts. Chem. Eur. J. 2012, 18, 10715–10732. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, C.J.; McInnis, J.P.; Chen, C.; Weberski, M.P.; Motta, A.; Delferro, M.; Marks, T.J. Ni(II) Phenoxyiminato Olefin Polymerization Catalysis: Striking Coordinative Modulation of Hyperbranched Polymer Microstructure and Stability by a Proximate Sulfonyl Group. ACS Catal. 2014, 4, 999–1003. [Google Scholar] [CrossRef]
- Ölscher, F.; Göttker-Schnetmann, I.; Monteil, V.; Mecking, S. Role of Radical Species in Salicylaldiminato Ni(II) Mediated Polymer Chain Growth: A Case Study for the Migratory Insertion Polymerization of Ethylene in the Presence of Methyl Methacrylate. J. Am. Chem. Soc. 2015, 137, 14819–14828. [Google Scholar] [CrossRef] [PubMed]
- Godin, A.; Göttker-Schnetmann, I.; Mecking, S. Nanocrystal Formation in Aqueous Insertion Polymerization. Macromolecules 2016, 49, 8825–8837. [Google Scholar] [CrossRef]
- Nakamura, A.; Anselment, T.M.J.; Claverie, J.; Goodall, B.; Jordan, R.F.; Mecking, S.; Rieger, B.; Sen, A.; van Leeuwen, P.W.N.M.; Nozaki, K. Ortho-phosphinobenzenesulfonate: A superb ligand for palladium-catalyzed coordination–insertion copolymerization of polar vinyl monomers. Acc. Chem. Res. 2013, 46, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Piche, L.; Daigle, J.C.; Rehse, G.; Claverie, J.P. Structure–Activity Relationship of Palladium Phosphanesulfonates: Toward Highly Active Palladium-Based Polymerization Catalysts. Chem. Eur. J. 2012, 18, 3277–3285. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Ito, S.; Kuroda, J.; Okumura, Y.; Nozaki, K. Quantification of the Steric Influence of Alkylphosphine–Sulfonate Ligands on Polymerization, Leading to High-Molecular-Weight Copolymers of Ethylene and Polar Monomers. J. Am. Chem. Soc. 2014, 136, 11898–11901. [Google Scholar] [CrossRef] [PubMed]
- Nakano, R.; Nozaki, K. Copolymerization of propylene and polar monomers using Pd/IzQO catalysts. J. Am. Chem. Soc. 2015, 137, 10934–10937. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.B.; Moritz, B.C.; Mecking, S. Suppression of Chain Transfer in Catalytic Acrylate Polymerization via Rapid and Selective Secondary Insertion. J. Am. Chem. Soc. 2015, 137, 2836–2839. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.B.; Mecking, S. Insertion Homo- and Copolymerization of Diallyl Ether. Angew. Chem. Int. Ed. 2015, 54, 15845–15849. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Cao, Y.C.; Leng, X.B.; Chen, C.; Huang, Z. Cationic Palladium(II) Complexes of Phosphine–Sulfonamide Ligands: Synthesis, Characterization, and Catalytic Ethylene Oligomerization. Organometallics 2014, 33, 3738–3745. [Google Scholar] [CrossRef]
- Sui, X.L.; Dai, S.Y.; Chen, C.L. Ethylene polymerization and copolymerization with polar monomers by cationic phosphine phosphonic amide palladium complexes. ACS Catal. 2015, 5, 5932–5937. [Google Scholar] [CrossRef]
- Chen, M.; Zou, W.P.; Cai, Z.G. Norbornene homopolymerization and copolymerization with ethylene by phosphine-sulfonate nickel catalysts. Polym. Chem. 2015, 6, 2669–2676. [Google Scholar] [CrossRef]
- Chen, M.; Yang, B.P.; Chen, C.L. Redox-controlled olefin (co)polymerization catalyzed by ferrocene-bridged phosphine-sulfonate palladium complexes. Angew. Chem. Int. Ed. 2015, 54, 15520–15744. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, B.P.; Chen, C.L. Redox Control in Olefin Polymerization and Copolymerization. Synlett 2016, 27, 1297–1302. [Google Scholar] [CrossRef]
- Na, Y.N.; Zhang, D.; Chen, C.L. Modulating the Polyolefin Properties through the Incorporation of Nitrogen-Containing Polar Monomers. Polym. Chem. 2017, 8, 2405–2409. [Google Scholar] [CrossRef]
- Jian, Z.; Falivene, L.; Boffa, G.; Ortega Sánchez, S.; Caporaso, L.; Grassi, A.; Mecking, S. Direct Synthesis of Telechelic Polyethylene by Selective Insertion Polymerization. Angew. Chem. Int. Ed. 2016, 55, 14378–14595. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Jordan, R.F. Olefin Insertion into a Pd–F Bond: Catalyst Reactivation Following β-F Elimination in Ethylene/Vinyl Fluoride Copolymerization. Angew. Chem. Int. Ed. 2017, 56, 1820–1824. [Google Scholar] [CrossRef] [PubMed]
- Rhinehart, J.L.; Brown, L.A.; Long, B.K. A robust Ni(II) α-diimine catalyst for high temperature ethylene polymerization. J. Am. Chem. Soc. 2013, 135, 16316–16319. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.Y.; Sui, X.L.; Chen, C.L. Highly Robust Palladium(II) α-Diimine Catalysts for Slow-Chain-Walking Polymerization of Ethylene and Copolymerization with Methyl Acrylate. Angew. Chem. Int. Ed. 2015, 54, 9948–9953. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.Y.; Chen, C.L. Direct Synthesis of Functionalized High-Molecular-Weight Polyethylene by Copolymerization of Ethylene with Polar Monomers. Angew. Chem. Int. Ed. 2016, 55, 13281–13285. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.Y.; Zhou, S.X.; Zhang, W.; Chen, C.L. Systematic Investigations of Ligand Steric Effects on α-Diimine Palladium Catalyzed Olefin Polymerization and Copolymerization. Macromolecules 2016, 49, 8855–8862. [Google Scholar] [CrossRef]
- Hu, X.H.; Dai, S.Y.; Chen, C.L. Ethylene polymerization by salicylaldimine nickel(II) complexes containing a dibenzhydryl moiety. Dalton Trans. 2016, 45, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Guironnet, D.; Roesle, P.; Runzi, T.; Gottker-Schnetmann, I.; Mecking, S. Insertion polymerization of acrylate. J. Am. Chem. Soc. 2009, 131, 422–423. [Google Scholar] [CrossRef] [PubMed]
- Perrotin, P.; McCahill, J.S.J.; Wu, G.; Scott, S.L. Linear, high molecular weight polyethylene from a discrete, mononuclear phosphinoarenesulfonate complex of nickel(II). Chem. Commun. 2011, 47, 6948–6950. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hu, H.; Xu, Y.; Guo, L.; Zai, S.; Song, K.; Gao, H.; Zhang, L.; Zhu, F.; Wu, Q. Thermostable α-Diimine Nickel(II) Catalyst for Ethylene Polymerization: Effects of the Substituted Backbone Structure on Catalytic Properties and Branching Structure of Polyethylene. Macromolecules 2009, 42, 7789–7796. [Google Scholar] [CrossRef]
- Guo, L.; Gao, H.; Guan, Q.; Hu, H.; Deng, J.; Liu, J.; Liu, F.; Wu, Q. Substituent effects of the backbone in α-diimine palladium catalysts on homo-and copolymerization of ethylene with methyl acrylate. Organometallics 2012, 31, 6054–6062. [Google Scholar] [CrossRef]
- Zou, W.P.; Chen, C.L. Influence of backbone substituents on the ethylene (co)polymerization properties of α-diimine Pd (II) and Ni (II) catalysts. Organometallics 2016, 35, 1794–1801. [Google Scholar] [CrossRef]
- Ota, Y.; Ito, S.; Kobayashi, M.; Kitade, S.; Sakata, K.; Tayano, T.; Nozaki, K. Crystalline Isotactic Polar Polypropylene from the Palladium-Catalyzed Copolymerization of Propylene and Polar Monomers. Angew. Chem. Int. Ed. 2016, 55, 7505–7509. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.X.; Chen, M.; Chen, C.L. Ethylene Polymerization and Copolymerization by Palladium and Nickel Catalysts Containing Naphthalene-Bridged Phosphine–Sulfonate Ligands. Organometallics 2016, 35, 1472–1479. [Google Scholar] [CrossRef]
- Nowack, R.J.; Hearley, A.K.; Rieger, B. New Phenylnickel-(2-phosphinobenzenesulfonate) Triphenylphosphine Complexes as Highly Active Ethylene Polymerization Catalysts. Anorg. Allg. Chem. 2005, 631, 2775–2781. [Google Scholar] [CrossRef]
- Ito, S.; Munakata, K.; Nakamura, A.; Nozaki, K. Copolymerization of Vinyl Acetate with Ethylene by Palladium/Alkylphosphine–Sulfonate Catalysts. J. Am. Chem. Soc. 2009, 131, 14606–14607. [Google Scholar] [CrossRef] [PubMed]
- Long, B.K.; Eagan, J.M.; Mulzer, M.; Coates, G.W. Semi-Crystalline Polar Polyethylene: Ester-Functionalized Linear Polyolefins Enabled by a Functional-Group-Tolerant, Cationic Nickel Catalyst. Z. Angew. Chem. Int. Ed. 2016, 55, 7222–7226. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C.L. Rational Design of High-Performance Phosphine Sulfonate Nickel Catalysts for Ethylene Polymerization and Copolymerization with Polar Monomers. ACS Catal. 2017, 7, 1308. [Google Scholar] [CrossRef]
- Cooper, C. Organic Chemist’s Desk Reference, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 1995; p. 151. [Google Scholar]
- Runzi, T.; Tritschler, U.; Roesle, P.; Gottker-Schnetmann, I.; Moller, H.M.; Caporaso, L.; Poater, A.; Cavallo, L.; Mecking, S. Activation and Deactivation of Neutral Palladium(II) Phosphinesulfonato Polymerization Catalysts. Organometallics 2012, 31, 8388–8408. [Google Scholar] [CrossRef]





| Entry | Catalyst | [cat] (μmol) | T (°C) | Yield (g) | Activity b | Mw c | Polydispersity c | Tm (°C) d |
|---|---|---|---|---|---|---|---|---|
| 1 | Pd1 | 10 | 80 | 2 | 2.0 | 2100 | 1.15 | 109 |
| 2 | Pd2 | 10 | 80 | 2.4 | 2.4 | 5100 | 2.36 | 117 |
| 3 | Pd3 | 10 | 80 | 5 | 5.0 | 27,800 | 2.56 | 129 |
| 4 | Pd1 | 2 | 80 | 0.5 | 2.5 | 3200 | 2.06 | 114 |
| 5 | Pd2 | 2 | 80 | 0.4 | 2.0 | 5700 | 2.18 | 124 |
| 6 | Pd3 | 2 | 80 | 1.2 | 6.0 | 24,800 | 1.84 | 131 |
| 7 | Ni1 | 10 | 80 | 1.9 | 1.9 | 3600 | 1.58 | 128 |
| 8 | Ni2 | 10 | 80 | 2.2 | 2.2 | 9100 | 2.18 | 129 |
| 9 | Ni3 | 10 | 80 | 5.7 | 5.7 | 17,500 | 2.04 | 133 |
| 10 | Ni1 | 2 | 25 | 0.7 | 1.7 | 37,200 | 3.64 | 134 |
| 11 | Ni2 | 2 | 25 | 0.5 | 1.3 | 58,600 | 1.99 | 135 |
| 12 | Ni3 | 2 | 25 | 1.4 | 3.5 | 142,300 | 1.51 | 136 |
| 13 | Pd2′ | 10 | 80 | 2.1 | 2.1 | 4200 | 2.28 | 123 |
| 14 | Pd2″ | 10 | 80 | 1.4 | 1.4 | 1800 | 1.44 | 115 |
| 15 | Ni2′ | 10 | 80 | 1.5 | 1.5 | 4100 | 1.46 | 124 |
| 16 | Ni2′ | 2 | 25 | 0.4 | 2.0 | 27,800 | 2.57 | 133 |
| 17 | Ni1″ | 10 | 80 | trace | - | - | - | - |
| 18 | Ni1″ | 2 | 25 | trace | - | - | - | - |
| Entry | Catalyst | P (bar) | T (oC) | Comonomer | [M] mol/L | Yield (mg) | Activity b | X c (%) | Mw d | Polydispersity d |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pd1 | 9 | 80 |  | 1.2 | 500 | 25 | 3 | 1000 | 1.19 |
| 2 | Pd2 | 9 | 80 |  | 1.2 | 340 | 17 | 5 | 4400 | 1.92 |
| 3 | Pd3 | 9 | 80 |  | 1.2 | 950 | 47 | 15 | 5500 | 1.55 |
| 4 | Pd1 | 9 | 80 |  | 2.5 | 200 | 10 | 8 | 1600 | 1.43 |
| 5 | Pd2 | 9 | 80 |  | 2.5 | 300 | 15 | 12 | 2100 | 1.35 |
| 6 | Pd3 | 9 | 80 |  | 2.5 | 510 | 25 | 27 | 3600 | 1.58 |
| 7 | Ni3 | 9 | 25 |  | 1.0 | 150 | 7.5 | 1.5 | 61,500 | 2.64 |
| 8 | Ni3 | 9 | 25 |  | 1.0 | 500 | 25 | 0.5 | 124,600 | 2.25 |
| 9 | Pd2″ | 9 | 25 |  | 1.2 | 110 | 5.5 | 2.5 | 1950 | 1.95 |
| 10 | Pd2″ | 9 | 25 |  | 2.5 | 80 | 4 | 6 | 1100 | 1.63 |
| 11 | Ni1″ | 9 | 25 |  | 1.0 | 0 | 0 | - | - | - |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Hong, C.; Du, H.; Pang, W.; Chen, C. Influence of Ligand Backbone Structure and Connectivity on the Properties of Phosphine-Sulfonate Pd(II)/Ni(II) Catalysts. Polymers 2017, 9, 168. https://doi.org/10.3390/polym9050168
Wu Z, Hong C, Du H, Pang W, Chen C. Influence of Ligand Backbone Structure and Connectivity on the Properties of Phosphine-Sulfonate Pd(II)/Ni(II) Catalysts. Polymers. 2017; 9(5):168. https://doi.org/10.3390/polym9050168
Chicago/Turabian StyleWu, Zixia, Changwen Hong, Hongxu Du, Wenmin Pang, and Changle Chen. 2017. "Influence of Ligand Backbone Structure and Connectivity on the Properties of Phosphine-Sulfonate Pd(II)/Ni(II) Catalysts" Polymers 9, no. 5: 168. https://doi.org/10.3390/polym9050168
APA StyleWu, Z., Hong, C., Du, H., Pang, W., & Chen, C. (2017). Influence of Ligand Backbone Structure and Connectivity on the Properties of Phosphine-Sulfonate Pd(II)/Ni(II) Catalysts. Polymers, 9(5), 168. https://doi.org/10.3390/polym9050168