Facile Synthesis and Self-Assembly of Amphiphilic Polyether-Octafunctionalized Polyhedral Oligomeric Silsesquioxane via Thiol-Ene Click Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
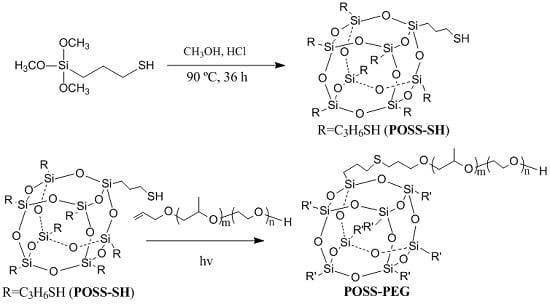
2.2. Synthesis of Octamercaptopropyl-POSS (POSS–SH)
2.3. Synthesis of Polyether-Octafunctionalized POSS (POSS–PEG)
2.4. Characterizations
2.5. Critical Micelle Concentration (CMC) Measurements
2.6. Dynamic Light Scattering (DLS)
3. Results and Discussion
3.1. Characterization of Octamercaptopropyl-POSS (POSS–SH)
3.2. Characterization of Amphiphilic Polymers
3.3. Self-Assembly Behaviour of Polymers
3.4. Thermal Response Aggregation Behavior
3.5. Thermal Stability Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, A.B.; Martinez-Manez, R.; Sancenon, R.; Hoffmann, K.; Rurack, K. The supramolecular chemistry of organic–inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 5924–5948. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic–inorganic hybrid nanocomposite-based gas sensors for environmental monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Sharma, S.; Dutta, S.; Zboril, R.; Gawande, M.B. Silica-nanosphere-based organic–inorganic hybrid nanomaterials: Synthesis, functionalization and applications in catalysis. Green Chem. 2015, 17, 3207–3230. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Lin, C.; Zhao, N. The in vitro bioactive of sol–gel bioactive glass powders with three-dimensional lamellar structure. Adv. Powder Technol. 2012, 23, 13–15. [Google Scholar] [CrossRef]
- Costa, D.O.; Dixon, S.J.; Rizkalla, A.S. One- and three-dimensional growth of hydroxyapatite nanowires during sol–gel-hydrothermal synthesis. ACS Appl. Mater. Interfaces 2012, 4, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Pan, A.; Wu, H.B.; Yu, L.; Zhu, T.; Lou, X.W. Synthesis of hierarchical three-dimensional vanadium oxide microstructures as high-capacity cathode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 3874–3879. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Lee, J.S.; Ahn, H.J.; Song, H.K.; Jang, J.H. Facile route to an efficient nio supercapacitor with a three-dimensional nanonetwork morphology. ACS Appl. Mater. Interfaces 2013, 5, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, E.; Kleebe, H.J.; Riedel, R. Silicon-containing polymer-derived ceramic nanocomposites (PDC-NCs): Preparative approaches and properties. Chem. Soc. Rev. 2012, 41, 5032–5052. [Google Scholar] [CrossRef] [PubMed]
- Mera, G.; Gallei, M.; Bernard, S.; Ionescu, E. Ceramic nanocomposites from tailor-made preceramic polymers. Nanomaterials 2015, 5, 468–540. [Google Scholar] [CrossRef] [PubMed]
- Bhandavat, R.; Kuhn, W.; Mansfield, E.; Lehman, J.; Singh, G. Synthesis of polymer-derived ceramic Si(B) CN-carbon nanotube composite by microwave-induced interfacial polarization. ACS Appl. Mater. Interfaces 2011, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Chang, L.Y.; Lim, S.K.; Zhao, J.; Smith, M.; Zhao, N.; Bulovic, V.; Bawendi, M.; Gradecak, S. Inorganic–organic hybrid solar cell: Bridging quantum dots to conjugated polymer nanowires. Nano Lett. 2011, 11, 3998–4002. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Ford, W.T.; Materer, N.F.; Teeters, D. Facile conversion of colloidal crystals to ordered porous polymer nets. J. Am. Chem. Soc. 2000, 122, 10472–10473. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.S.; Kim, K.H.; Jin, Y.H.; Hong, S.M.; San Hwang, S.; Cho, B.G.; Shin, D.Y.; Im, S.S. Applications of telechelic polymers as compatibilizers and stabilizers in polymer blends and inorganic/organic nanohybrids. Polymer 2004, 45, 3527–3533. [Google Scholar] [CrossRef]
- Wang, F.K.; Lu, X.H.; He, C.B. Some recent developments of polyhedral oligomeric silsesquioxane (POSS)-based polymeric materials. J. Mater. Chem. 2011, 21, 2775–2782. [Google Scholar] [CrossRef]
- Ghanbari, H.; Cousins, B.G.; Seifalian, A.M. A nanocage for nanomedicine: Polyhedral oligomeric silsesquioxane (POSS). Macromol. Rapid Commun. 2011, 32, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral oligomeric silsesquioxane (POSS)-containing polymer nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jin, J.; Song, M.; Shaw, S.; Stone, C. Curing dynamics and network formation of cyanate ester resin/polyhedral oligomeric silsesquioxane nanocomposites. Polymer 2011, 52, 1716–1724. [Google Scholar] [CrossRef]
- Geng, Z.; Huo, M.; Mu, J.; Zhang, S.; Lu, Y.; Luan, J.; Huo, P.; Du, Y.; Wang, G. Ultra low dielectric constant soluble polyhedral oligomeric silsesquioxane (POSS)–poly (aryl ether ketone) nanocomposites with excellent thermal and mechanical properties. J. Mater. Chem. C 2014, 2, 1094–1103. [Google Scholar] [CrossRef]
- Li, Z.; Wu, D.; Liang, Y.; Fu, R.; Matyjaszewski, K. Synthesis of well-defined microporous carbons by molecular-scale templating with polyhedral oligomeric silsesquioxane moieties. J. Am. Chem. Soc. 2014, 136, 4805–4808. [Google Scholar] [CrossRef] [PubMed]
- Fatieiev, Y.; Croissant, J.; Julfakyan, K.; Deng, L.; Anjum, D.H.; Gurinov, A.; Khashab, N. Enzymatically degradable hybrid organic–inorganic bridged silsesquioxane nanoparticles for in vitro imaging. Nanoscale 2015, 7, 15046–15050. [Google Scholar] [CrossRef] [PubMed]
- Raftopoulos, K.N.; Pielichowski, K. Segmental dynamics in hybrid polymer/POSS nanomaterials. Prog. Polym. Sci. 2015, 7, 15046–15050. [Google Scholar] [CrossRef]
- Wu, J.; Mather, P.T. Poss polymers: Physical properties and biomaterials applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Croissant, J.G.; Cattoen, X.; Durand, J.O.; Man, M.W.C.; Khashab, N.M. Organosilica hybrid nanomaterials with a high organic content: Syntheses and applications of silsesquioxanes. Nanoscale 2016, 8, 19945–19972. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, H.L.; Tam, A.Y.Y.; Leung, S.Y.L.; Yam, V.W.W. Supramolecular assembly of platinum-containing polyhedral oligomeric silsesquioxanes: An interplay of intermolecular interactions and a correlation between structural modifications and morphological transformations. Chem. Sci. 2017, 8, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, A.S.; Lee, J.C.; Hong, S.M.; Hwang, S.S.; Koo, C.M. Multifunctional mesoporous ionic gels and scaffolds derived from polyhedral oligomeric silsesquioxanes. ACS Appl. Mater. Interfaces 2017, 9, 3616–3623. [Google Scholar] [CrossRef] [PubMed]
- Schafer, S.; Kickelbick, G. Simple and high yield access to octafunctional azido, amine and urea group bearing cubic spherosilicates. Dalton Trans. 2017, 46, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J. Nanotechnology applications in medicine and dentistry. J. Investig. Clin. Dent. 2011, 2, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.; Noda, A.; Nishimoto, A.; Watanabe, M. Xps study of lithium surface after contact with lithium-salt doped polymer electrolytes. Electrochim. Acta 2001, 46, 1595–1603. [Google Scholar] [CrossRef]
- Kim, B.S.; Mather, P.T. Amphiphilic telechelics incorporating polyhedral oligosilsesquioxane: 1. Synthesis and characterization. Macromolecules 2002, 35, 8378–8384. [Google Scholar] [CrossRef]
- Kim, B.S.; Mather, P.T. Morphology, microstructure, and rheology of amphiphilic telechelics incorporating polyhedral oligosilsesquioxane. Macromolecules 2006, 39, 9253–9260. [Google Scholar] [CrossRef]
- Kim, B.S.; Mather, P.T. Amphiphilic telechelics with polyhedral oligosilsesquioxane (POSS) end-groups: Dilute solution viscometry. Polymer 2006, 47, 6202–6207. [Google Scholar] [CrossRef]
- Maitra, P.; Wunder, S.L. Oligomeric poly(ethylene oxide)-functionalized silsesquioxanes: Interfacial effects on Tg, Tm, and ΔHm. Chem. Mater. 2002, 14, 4494–4497. [Google Scholar] [CrossRef]
- Lee, W.; Ni, S.L.; Deng, J.J.; Kim, B.S.; Satija, S.K.; Mather, P.T.; Esker, A.R. Telechelic poly(ethylene glycol)–POSS amphiphiles at the air/water interface. Macromolecules 2007, 40, 682–688. [Google Scholar] [CrossRef]
- Markovic, E.; Ginic-Markovic, M.; Clarke, S.; Matisons, J.; Hussain, M.; Simon, G.P. Poly(ethylene glycol)-octafunctionalized polyhedral oligomeric silsesquioxane: Synthesis and thermal analysis. Macromolecules 2007, 40, 2694–2701. [Google Scholar] [CrossRef]
- Markovic, E.; Matisons, J.; Hussain, M.; Simon, G.P. Poly(ethylene glycol) octafunctionalized polyhedral oligomeric silsesquioxane: Waxd and rheological studies. Macromolecules 2007, 40, 4530–4534. [Google Scholar] [CrossRef]
- Miao, J.J.; Cui, L.; Lau, H.P.; Mather, P.T.; Zhu, L. Self-assembly and chain-folding in hybrid coil-coil-cube triblock oligomers of polyethylene–b–poly(ethylene oxide)–b–polyhedral oligomeric silsesquioxane. Macromolecules 2007, 40, 5460–5470. [Google Scholar] [CrossRef]
- Mu, J.F.; Liu, Y.H.; Zheng, S.X. Inorganic-organic interpenetrating polymer networks involving polyhedral oligomeric silsesquioxane and poly(ethylene oxide). Polymer 2007, 48, 1176–1184. [Google Scholar] [CrossRef]
- Li, Z.B.; Tan, B.H.; Jin, G.R.; Li, K.; He, C.B. Design of polyhedral oligomeric silsesquioxane (POSS) based thermo-responsive amphiphilic hybrid copolymers for thermally denatured protein protection applications. Polym. Chem. 2014, 5, 6740–6753. [Google Scholar] [CrossRef]
- Wang, D.K.; Varanasi, S.; Strounina, E.; Hill, D.J.T.; Symons, A.L.; Whittaker, A.K.; Rasoul, F. Synthesis and characterization of a POSS–PEG macromonomer and POSS–PEG–PLA hydrogels for periodontal applications. Biomacromolecules 2014, 15, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; Jheng, Y.R.; Yeh, S.L.; Chen, T.; Kuo, S.W. Unusual emission of polystyrene-based alternating copolymers incorporating aminobutyl maleimide fluorophore-containing polyhedral oligomeric silsesquioxane nanoparticles. Polymers 2017, 9, 103. [Google Scholar] [CrossRef]
- Mukbaniani, O.; Titvinidze, G.; Tatrishvili, T.; Mukbaniani, N.; Brostow, W.; Pietkiewicz, D. Formation of polymethylsiloxanes with alkyl side groups. J. Appl. Polym. Sci. 2007, 104, 1176–1183. [Google Scholar] [CrossRef]
- Li, Y.J.; Wan, L.Q.; Huang, F.R.; Du, L. Core–shell octa(azidopropyl) POSS–PEO micelle via “click” chemistry. J. Mol. Liq. 2014, 196, 238–243. [Google Scholar] [CrossRef]
- Xia, Y.; Yao, H.; Cui, M.; Ma, Y.; Kong, Z.; Wu, B.; Qi, Z.; Sun, Y. Theoretical and experimental investigations on mono-substituted and multi-substituted functional polyhedral oligomeric silsesquioxanes. RSC Adv. 2015, 5, 80339–80345. [Google Scholar] [CrossRef]
- Bakkour, Y.; Darcos, V.; Li, S.M.; Coudane, J. Diffusion ordered spectroscopy (DOSY) as a powerful tool for amphiphilic block copolymer characterization and for critical micelle concentration (CMC) determination. Polym. Chem. 2012, 3, 2006–2010. [Google Scholar] [CrossRef]
- Pineiro, L.; Novo, M.; Al-Soufi, W. Fluorescence emission of pyrene in surfactant solutions. Adv. Colloid Interface Sci. 2015, 215, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wang, D.X.; Yang, Z.Z.; Liang, Y.; Zhang, J.; Feng, S.Y. Facile, versatile and efficient synthesis of functional polysiloxanes via thiol-ene chemistry. Eur. Polym. J. 2013, 49, 1050–1056. [Google Scholar] [CrossRef]
- Shipp, D.A.; McQuinn, C.W.; Rutherglen, B.G.; McBath, R.A. Elastomeric and degradable polyanhydride network polymers by step-growth thiol-ene photopolymerization. Chem. Commun. 2009, 6415–6417. [Google Scholar] [CrossRef] [PubMed]
- Rissing, C.; Son, D.Y. Thiol-ene reaction for the synthesis of multifunctional branched organosilanes. Organometallics 2008, 27, 5394–5397. [Google Scholar] [CrossRef]
- Mya, K.Y.; Li, X.; Chen, L.; Ni, X.P.; Li, J.; He, C.B. Core-corona structure of cubic silsesquioxane-poly(ethylene oxide) in aqueous solution: Fluorescence, light scattering, and tem studies. J. Phys. Chem. B 2005, 109, 9455–9462. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Nazarova, I.R.; Astafieva, I.V.; Batrakova, E.V.; Alakhov, V.Y.; Yaroslavov, A.A.; Kabanov, V.A. Micelle formation and solubilization of fluorescent-probes in poly(oxyethylene-b-oxypropylene-b-oxyethylene) solutions. Macromolecules 1995, 28, 2303–2314. [Google Scholar] [CrossRef]
- Xia, Y.; Yao, H.T.; Miao, Z.H.; Ma, Y.; Cui, M.F.; Yan, L.Q.; Ling, H.H.; Qi, Z.J. Facile synthesis and self-assembly of amphiphilic polydimethylsiloxane with poly(ethylene glycol) moieties via thiol-ene click reaction. RSC Adv. 2015, 5, 50955–50961. [Google Scholar] [CrossRef]
- Chu, B. Laser Light Scattering: Basic Principles and Practice; Academic Press: New York, NY, USA, 1991. [Google Scholar]
- Hussain, H.; Tan, B.H.; Seah, G.L.; Liu, Y.; He, C.B.; Davis, T.P. Micelle formation and gelation of (PEG–P(MA-POSS)) amphiphilic block copolymers via associative hydrophobic effects. Langmuir 2010, 26, 11763–11773. [Google Scholar] [CrossRef] [PubMed]
- Louguet, S.; Rousseau, B.; Epherre, R.; Guidolin, N.; Goglio, G.; Mornet, S.; Duguet, E.; Lecommandoux, S.; Schatz, C. Thermoresponsive polymer brush-functionalized magnetic manganite nanoparticles for remotely triggered drug release. Polym. Chem. 2012, 3, 1408–1417. [Google Scholar] [CrossRef]
- Persson, J.; Kaul, A.; Tjerneld, F. Polymer recycling in aqueous two-phase extractions using thermoseparating ethylene oxide-propylene oxide copolymers. J. Chromatogr. B 2000, 743, 115–126. [Google Scholar] [CrossRef]











© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Ding, S.; Liu, Y.; Qi, Z. Facile Synthesis and Self-Assembly of Amphiphilic Polyether-Octafunctionalized Polyhedral Oligomeric Silsesquioxane via Thiol-Ene Click Reaction. Polymers 2017, 9, 251. https://doi.org/10.3390/polym9070251
Xia Y, Ding S, Liu Y, Qi Z. Facile Synthesis and Self-Assembly of Amphiphilic Polyether-Octafunctionalized Polyhedral Oligomeric Silsesquioxane via Thiol-Ene Click Reaction. Polymers. 2017; 9(7):251. https://doi.org/10.3390/polym9070251
Chicago/Turabian StyleXia, Yong, Sha Ding, Yuejun Liu, and Zhengjian Qi. 2017. "Facile Synthesis and Self-Assembly of Amphiphilic Polyether-Octafunctionalized Polyhedral Oligomeric Silsesquioxane via Thiol-Ene Click Reaction" Polymers 9, no. 7: 251. https://doi.org/10.3390/polym9070251
APA StyleXia, Y., Ding, S., Liu, Y., & Qi, Z. (2017). Facile Synthesis and Self-Assembly of Amphiphilic Polyether-Octafunctionalized Polyhedral Oligomeric Silsesquioxane via Thiol-Ene Click Reaction. Polymers, 9(7), 251. https://doi.org/10.3390/polym9070251