Poly(3,3-dibenzyl-3,4-dihydro-2H-thieno[3,4-b][1,4]dioxepine)/Platinum Composite Films as Potential Counter Electrodes for Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PProDOT-Bz2 Counter Electrodes
2.3. Assembly of the DSSCs
2.4. Characterization
3. Results and Discussion
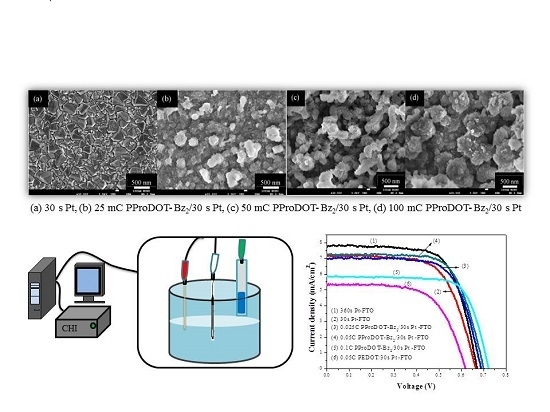
3.1. Surface Morphology of the PProDOT-Bz2 Film
3.2. Photovoltaic Performances
3.3. Electrochemical Impedance Spectra
3.4. Cyclic Voltammetry
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Tsao, M.H.; Wu, T.Y.; Wang, H.P.; Sun, I.W.; Su, S.G.; Lin, Y.C.; Chang, C.W. An efficient metal free sensitizer for dye-sensitized solar cells. Mater. Lett. 2011, 65, 583–586. [Google Scholar] [CrossRef]
- Che Balian, S.R.; Ahmad, A.; Mohamed, N.S. The effect of lithium iodide to the properties of carboxymethyl κ-carrageenan/carboxymethyl cellulose polymer electrolyte and Dye-sensitized solar cell performance. Polymers 2016, 8, 163. [Google Scholar] [CrossRef]
- Wu, T.Y.; Tsao, M.H.; Chen, F.L.; Su, S.G.; Chang, C.W.; Wang, H.P.; Lin, Y.C.; Ou-Yang, W.C.; Sun, I.W. Synthesis and characterization of organic dyes containing various donors and acceptors. Int. J. Mol. Sci. 2010, 11, 329–353. [Google Scholar] [CrossRef] [PubMed]
- Dzulkurnain, N.A.; Ahmad, A.; Mohamed, N.S. P(MMA–EMA) random copolymer electrolytes incorporating sodium iodide for potential application in a Dye-sensitized solar cell. Polymers 2015, 7, 266–280. [Google Scholar] [CrossRef]
- Wu, T.Y.; Tsao, M.H.; Chen, F.L.; Su, S.G.; Chang, C.W.; Wang, H.P.; Lin, Y.C.; Sun, I.W. Synthesis and characterization of three organic dyes with various donors and rhodanine ring acceptor for use in dye-sensitized solar cells. J. Iran. Chem. Soc. 2010, 7, 707–720. [Google Scholar] [CrossRef]
- Sun, I.W.; Wang, H.P.; Teng, H.; Su, S.G.; Lin, Y.C.; Kuo, C.W.; Chen, P.R.; Wu, T.Y. Cyclic ammonium-based ionic liquids as potential electrolytes for dye-sensitized solar cells. Int. J. Electrochem. Sci. 2012, 7, 9748–9764. [Google Scholar]
- Wu, T.Y.; Tsao, M.H.; Su, S.G.; Wang, H.P.; Lin, Y.C.; Chen, F.L.; Chang, C.W.; Sun, I.W. Synthesis, characterization and photovoltaic properties of di-anchoring organic dyes. J. Braz. Chem. Soc. 2011, 22, 780–789. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, A.R.; Madhavan, J.; Maiyalagan, T. Recent progress in non-platinum counter electrode materials for dye-sensitized solar cells. Chem. Electron. Chem. 2015, 2, 928–945. [Google Scholar] [CrossRef]
- Bora, C.; Sarkar, C.; Mohan, K.J.; Dolui, S. Polythiophene/graphene composite as a highly efficient platinum-free counter electrode in dye-sensitized solar cells. Electrochim. Acta 2015, 157, 225–231. [Google Scholar] [CrossRef]
- Yun, S.; Lund, P.D.; Hinsch, A. Stability assessment of alternative platinum free counter electrodes for dye-sensitized solar cells. Energy Environ. Sci. 2015, 8, 3495–3514. [Google Scholar] [CrossRef]
- Jeon, S.S.; Kim, C.; Lee, T.H.; Lee, Y.W.; Do, K.; Ko, J.; Im, S.S. Camphorsulfonic acid-doped polyaniline transparent counter electrode for dye-sensitized solar cells. J. Phys. Chem. C 2012, 116, 22743–22748. [Google Scholar] [CrossRef]
- Qiu, Y.; Lu, S.; Wang, S.; Zhang, X.; He, S.; He, T. High-performance polyaniline counter electrode electropolymerized in presence of sodium dodecyl sulfate for dye-sensitized solar cells. J. Power Sources 2014, 253, 300–304. [Google Scholar] [CrossRef]
- Chen, L.; Guo, C.X.; Zhang, Q.; Lei, Y.; Xie, J.; Ee, S.; Guai, G.; Song, Q.; Li, C.M. Graphene quantum-dot-doped polypyrrole counter electrode for high-performance dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.Y.; Koh, J.K.; Kim, J.K.; Lee, C.S.; Kim, J.H. Three-dimensional conducting polymer films for Pt-free counter electrodes in quasi-solid-state dye-sensitized solar cells. Electrochim. Acta 2014, 137, 34–40. [Google Scholar] [CrossRef]
- Kim, H.; Veerappan, G.; Park, J.H. Conducting polymer coated non-woven graphite fiber film for dye-sensitized solar cells: superior Pt-and FTO-free counter electrodes. Electrochim. Acta 2014, 137, 164–168. [Google Scholar] [CrossRef]
- Han, R.; Lu, S.; Wang, Y.; Zhang, X.; Wu, Q.; He, T. Influence of monomer concentration during polymerization on performance and catalytic mechanism of resultant poly(3,4-ethylenedioxythiophene) counter electrodes for dye-sensitized solar cells. Electrochim. Acta 2015, 173, 796–803. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Li, C.-T.; Lee, C.-P.; Vittal, R.; Ho, K.-C. PEDOT-decorated nitrogen-doped graphene as the transparent composite film for the counter electrode of a dye-sensitized solar cell. Nano Energy 2015, 12, 374–385. [Google Scholar] [CrossRef]
- Ke, C.-R.; Chang, C.-C.; Ting, J.-M. Modified conducting polymer films having high catalytic activity for use as counter electrodes in rigid and flexible dye-sensitized solar cells. J. Power Sources 2015, 284, 489–496. [Google Scholar] [CrossRef]
- Seo, H.; Son, M.-K.; Itagaki, N.; Koga, K.; Shiratani, M. Polymer counter electrode of poly(3,4-ethylenedioxythiophene):poly(4-styrenesulfonate) containing TiO2 nano-particles for dye-sensitized solar cells. J. Power Sources 2016, 307, 25–30. [Google Scholar] [CrossRef]
- Yeh, M.-H.; Lee, C.-P.; Lin, L.-Y.; Nien, P.-C.; Chen, P.-Y.; Vittal, R.; Ho, K.-C. A composite poly(3,3-diethyl-3,4-dihydro-2H-thieno-[3,4-b][1,4]-dioxepine) and Pt film as a counter electrode catalyst in dye-sensitized solar cells. Electrochim. Acta 2011, 56, 6157–6164. [Google Scholar] [CrossRef]
- Kang, G.; Choi, J.; Park, T. Pt-free counter electrodes with carbon black and 3D network epoxy polymer composites. Sci. Rep. 2016, 6, 22987. [Google Scholar] [CrossRef] [PubMed]
- Bella, F.; Galliano, S.; Falco, M.; Viscardi, G.; Barolo, C.; Gratzel, M.; Gerbaldi, C. Approaching truly sustainable solar cells by the use of water and cellulose derivatives. Green Chem. 2017, 19, 1043–1051. [Google Scholar] [CrossRef]
- Bella, F.; Pugliese, D.; Zolin, L.; Gerbaldi, C. Paper-based quasi-solid dye-sensitized solar cells. Electrochim. Acta 2017, 237, 87–93. [Google Scholar] [CrossRef]
- Gemeiner, P.; Peřinka, N.; Švorc, L.; Hatala, M.; Gál, L.; Belovičová, M.; Syrový, T.; Mikula, M. Pt–free counter electrodes based on modified screen-printed PEDOT:PSS catalytic layers for dye-sensitized solar cells. Mater. Sci. Semicond. Process. 2017, 66, 162–169. [Google Scholar] [CrossRef]
- Gerosa, M.; Sacco, A.; Scalia, A.; Bella, F.; Chiodoni, A.; Quaglio, M.; Tresso, E.; Bianco, S. Toward totally flexible Dye-sensitized solar cells based on titanium grids and polymeric electrolyte. IEEE J. Photovolt. 2016, 6, 498–505. [Google Scholar] [CrossRef]
- Imperiyka, M.; Ahmad, A.; Hanifah, S.A.; Bella, F. A UV-prepared linear polymer electrolyte membrane for dye-sensitized solar cells. Phys. Rev. B 2014, 450, 151–154. [Google Scholar] [CrossRef]
- Pavithra, N.; Velayutham, D.; Sorrentino, A.; Anandan, S. Thiourea incorporated poly(ethylene oxide) as transparent gel polymer electrolyte for dye sensitized solar cell applications. J. Power Sources 2017, 353, 245–253. [Google Scholar] [CrossRef]
- Shanti, R.; Bella, F.; Salim, Y.S.; Chee, S.Y.; Ramesh, S.; Ramesh, K. Poly(methyl methacrylate–co–butyl acrylate–co–acrylic acid): Physico–chemical characterization and targeted dye sensitized solar cell application. Mater. Des. 2016, 108, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.; Song, D.; Lee, Y.-G.; Son, T.; Cho, W.; Pyun, Y.B.; Kim, T.-Y.; Lee, J.H.; Fabregat-Santiago, F.; Bisquert, J.; et al. Chemical effects of tin oxide nanoparticles in polymer electrolytes-based Dye-sensitized solar cells. J. Phys. Chem. C 2014, 118, 16510–16517. [Google Scholar] [CrossRef]
- Wu, T.Y.; Liao, J.W.; Chen, C.Y. Electrochemical synthesis, characterization and electrochromic properties of indan and 1,3-benzodioxole-based poly(2,5-dithienylpyrrole) derivatives. Electrochim. Acta 2014, 150, 245–262. [Google Scholar] [CrossRef]
- Wu, T.Y.; Su, Y.S. Electrochemical synthesis and characterization of a 1,4-benzodioxan-based electrochromic polymer and its application in electrochromic devices. J. Electrochem. Soc. 2015, 162, 103–112. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chung, H.H. Applications of tris(4-(thiophen-2-yl)phenyl)amine-and dithienylpyrrole-based conjugated copolymers in high-contrast electrochromic devices. Polymers 2016, 8, 206. [Google Scholar] [CrossRef]
- Chou, J.-C.; Lin, S.-C.; Liao, Y.-H.; Hu, J.-E.; Chuang, S.-W.; Huang, C.-H. The influence of electrophoretic deposition for fabricating dye-sensitized solar cell. J. Nanomater. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Li, C.-T.; Lee, C.-T.; Li, S.-R.; Lee, C.-P.; Chiu, I.T.; Vittal, R.; Wu, N.-L.; Sun, S.-S.; Ho, K.-C. Composite films of carbon black nanoparticles and sulfonated-polythiophene as flexible counter electrodes for dye-sensitized solar cells. J. Power Sources 2016, 302, 155–163. [Google Scholar] [CrossRef]





| Counter Electrode | Voc (V) | Jsc (mA/cm2) | F.F. (%) | η (%) |
|---|---|---|---|---|
| 360 s Pt | 0.67 ± 0.02 | 7.80 ± 0.25 | 68.61 ± 2.77 | 3.58 ± 0.10 |
| 30 s Pt | 0.67 ± 0.03 | 7.20 ± 0.31 | 64.94 ± 2.34 | 3.11 ± 0.11 |
| 0.025 C/cm2 PProDOT-Bz2/30 s Pt | 0.69 ± 0.02 | 7.03 ± 0.45 | 68.82 ± 2.90 | 3.33 ± 0.11 |
| 0.05 C/cm2 PProDOT-Bz2/30 s Pt | 0.70 ± 0.04 | 7.27 ± 0.35 | 68.74 ± 2.25 | 3.50 ± 0.13 |
| 0.1 C/cm2 PProDOT-Bz2/30 s Pt | 0.62 ± 0.03 | 5.31 ± 0.33 | 64.46 ± 2.83 | 2.08 ± 0.13 |
| 0.05 C/cm2 PEDOT/30 s Pt | 0.72 ± 0.04 | 5.88 ± 0.28 | 73.09 ± 2.14 | 3.10 ± 0.14 |
| Counter Electrode | Rs (Ω) | C1 (µF) | R1(Ω) | C2 (mF) | R2 (Ω) |
|---|---|---|---|---|---|
| 360 s Pt–FTO | 18.42 | 85.63 | 1.05 | 5.12 | 12.57 |
| 30 s Pt–FTO | 21.65 | 27.49 | 7.40 | 3.61 | 15.71 |
| 0.025 C/cm2 PProDOT-Bz2/30 s Pt–FTO | 17.82 | 38.05 | 1.65 | 3.89 | 12.43 |
| 0.05 C/cm2 PProDOT-Bz2/30 s Pt–FTO | 17.43 | 66.63 | 2.08 | 3.49 | 10.57 |
| 0.1 C/cm2 PProDOT-Bz2/30 s Pt–FTO | 26.85 | 0.013 | 15.14 | 3.04 | 51.47 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, J.-C.; Huang, Y.-C.; Wu, T.-Y.; Liao, Y.-H.; Lai, C.-H.; Chu, C.-M.; Lin, Y.-J. Poly(3,3-dibenzyl-3,4-dihydro-2H-thieno[3,4-b][1,4]dioxepine)/Platinum Composite Films as Potential Counter Electrodes for Dye-Sensitized Solar Cells. Polymers 2017, 9, 271. https://doi.org/10.3390/polym9070271
Chou J-C, Huang Y-C, Wu T-Y, Liao Y-H, Lai C-H, Chu C-M, Lin Y-J. Poly(3,3-dibenzyl-3,4-dihydro-2H-thieno[3,4-b][1,4]dioxepine)/Platinum Composite Films as Potential Counter Electrodes for Dye-Sensitized Solar Cells. Polymers. 2017; 9(7):271. https://doi.org/10.3390/polym9070271
Chicago/Turabian StyleChou, Jung-Chuan, Yu-Chi Huang, Tzi-Yi Wu, Yi-Hung Liao, Chih-Hsien Lai, Chia-Ming Chu, and Yu-Jen Lin. 2017. "Poly(3,3-dibenzyl-3,4-dihydro-2H-thieno[3,4-b][1,4]dioxepine)/Platinum Composite Films as Potential Counter Electrodes for Dye-Sensitized Solar Cells" Polymers 9, no. 7: 271. https://doi.org/10.3390/polym9070271
APA StyleChou, J. -C., Huang, Y. -C., Wu, T. -Y., Liao, Y. -H., Lai, C. -H., Chu, C. -M., & Lin, Y. -J. (2017). Poly(3,3-dibenzyl-3,4-dihydro-2H-thieno[3,4-b][1,4]dioxepine)/Platinum Composite Films as Potential Counter Electrodes for Dye-Sensitized Solar Cells. Polymers, 9(7), 271. https://doi.org/10.3390/polym9070271