Stacking Resistance Genes in Multiparental Interspecific Potato Hybrids to Anticipate Late Blight Outbreaks
Abstract
:1. Introduction
2. Materials & Methods
2.1. Plant Material
2.2. Resistance to Pathogens
2.3. Molecular and Bioinformatics Methods
2.4. SCAR Markers for Resistance Genes
3. Results & Discussion
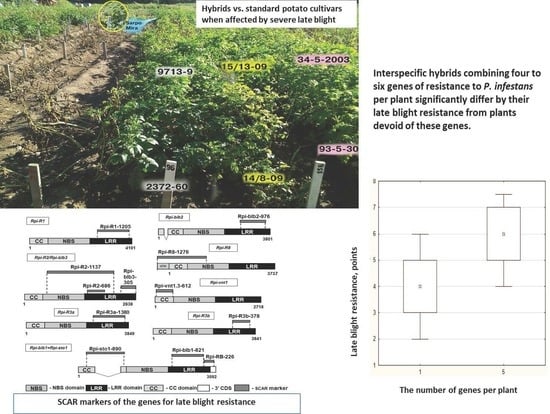
3.1. LB Resistance of the Multiparental Potato Hybrids
3.2. Rpi Genes in the Multiparental Potato Hybrids
3.3. LB Resistance is Enhanced by Pyramiding Rpi Genes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooke, D.E.L.; Cano, L.M.; Raffaele, S.; Bain, R.A.; Cooke, L.R.; Etherington, G.J.; Deahl, K.L.; Farrer, R.A.; Gilroy, E.M.; Goss, E.M.; et al. Genome analyses of an aggressive and invasive lineage of the irish potato famine pathogen. PLoS Pathog. 2012, 8, e1002940. [Google Scholar] [CrossRef]
- Fry, W.E. Phytophthora infestans: New tools (and old ones) lead to new understanding and precision management. Ann. Rev. Phytopathol. 2016, 54, 529–547. [Google Scholar] [CrossRef]
- Haverkort, A.J.; Boonekamp, P.M.; Hutten, R.; Jacobsen, E.; Lotz, L.A.P.; Kessel, G.J.T.; Vossen, J.H.; Visser, R.G.F. Durable late blight resistance in potato through dynamic varieties obtained by cisgenesis: Scientific and societal advances in the durph project. Potato Res. 2016, 59, 35–66. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.K.M. Durable resistance of crops to disease: A darwinian perspective. Ann. Rev. Phytopathol. 2015, 53, 513–539. [Google Scholar] [CrossRef]
- Gebhardt, C.; Bellin, D.; Henselewski, H.; Lehmann, W.; Schwarzfischer, J.; Valkonen, J.P.T. Marker-assisted combination of major genes for pathogen resistance in potato. Theor. Appl. Genet. 2006, 112, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.E. Review and analysis of limitations in ways to improve conventional potato breeding. Potato Res. 2017, 60, 171–193. [Google Scholar] [CrossRef]
- Vossen, J.H.; Jo, K.-R.; Vosman, B. Mining the genus Solanum for increasing disease resistance. In Genomics of Plant Genetic Resources; Tuberosa, R., Graner, A., Frison, E., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 27–46. [Google Scholar]
- Haesaert, G.; Vossen, J.H.; Custers, R.; De Loose, M.; Haverkort, A.; Heremans, B.; Hutten, R.; Kessel, G.; Landschoot, S.; Van Droogenbroeck, B.; et al. Transformation of the potato variety Desiree with single or multiple resistance genes increases resistance to late blight under field conditions. Crop. Prot. 2015, 77, 163–175. [Google Scholar] [CrossRef]
- Hardigan, M.A.; Laimbeer, F.P.E.; Newton, L.; Crisovan, E.; Hamilton, J.P.; Vaillancourt, B.; Wiegert-Rininger, K.; Wood, J.C.; Douches, D.S.; Farré, E.M.; et al. Genome diversity of tuber-bearing Solanum uncovers complex evolutionary history and targets of domestication in the cultivated potato. Proc. Natl. Acad. Sci. USA 2017, 114, E9999–E10008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bethke, P.C.; Halterman, D.; Jansky, S. Potato germplasm enhancement enters the genomics era. Agronomy 2019, 9, 575. [Google Scholar] [CrossRef] [Green Version]
- Gaiero, P.; Speranza, P.R.; De Jong, H. Introgressive hybridization in potato revealed by novel cytogenetic and genomic technologies. Am. J. Potato Res. 2018, 95, 607–621. [Google Scholar] [CrossRef] [Green Version]
- McDonald, B.A.; Linde, C. Pathogen population genetics, Evolutionary potential, and durable resistance. Ann. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundt, C.C. Durable resistance: A key to sustainable management of pathogens and pests. Infect. Genet. Evol. 2014, 27, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Mundt, C.C. Pyramiding for resistance durability: theory and practice. Phytopathology 2018, 108, 792–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stam, R.; McDonald, B.A. When resistance gene pyramids are not durable-the role of pathogen diversity. Mol. Plant Pathol. 2018, 19, 521–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wersch, S.; Li, X. Stronger when together: Clustering of plant nlr disease resistance genes. Trends Plant Sci. 2019, 24, 688–699. [Google Scholar] [CrossRef]
- Halterman, D.A.; Jansky, S.H.; Spooner, D.M. Discovery of new sources of disease resistance using wild potato germplasm. Am. J. Potato Res. 2017, 94, 211–250. [Google Scholar] [CrossRef]
- Rogozina, E.V.; Khavkin, E.E. Interspecific potato hybrids as donors of durable resistance to pathogens. Vavilov J. Genet. Breed. 2017, 21, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Colleoni, C.; Zhang, J.; Liang, Q.; Hu, Y.; Ruess, H.; Simon, R.; Liu, Y.; Liu, H.; Yu, G.; et al. Genomic analyses yield markers for identifying agronomically important genes in potato. Mol. Plant 2018, 11, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Karki, H.S.; Jansky, S.H.; Halterman, D.A. Screening of wild potatoes identifies new sources of late blight resistance. Plant Dis. 2020. [Google Scholar] [CrossRef]
- Sokolova, E.; Pankin, A.; Beketova, M.; Kuznetsova, M.; Spiglazova, S.; Rogozina, E.V.; Yashina, I.; Khavkin, E. SCAR markers of the R-genes and germplasm of wild Solanum species for breeding late blight-resistant potato cultivars. Plant Genet. Res. 2011, 9, 309–312. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Siddappa, S.; Singh, B.P.; Kaushik, S.K.; Chakrabarti, S.K.; Bhardwaj, V.; Chandel, P. Molecular markers for late blight resistance breeding of potato: An update. Plant Breed. 2013, 132, 237–245. [Google Scholar] [CrossRef]
- Ramakrishnan, A.P.; Ritland, C.E.; Sevillano, R.H.B.; Riseman, A. Review of potato molecular markers to enhance trait selection. Am. J. Potato Res. 2015, 92, 455–472. [Google Scholar] [CrossRef]
- Jupe, F.; Witek, K.; Verweij, W.; Śliwka, J.; Pritchard, L.; Etherington, G.J.; MacLean, D.; Cock, P.J.; Leggett, R.M.; Bryan, G.J.; et al. Resistance gene enrichment sequencing (RenSeq) enables reannotation of the NB-LRR gene family from sequenced plant genomes and rapid mapping of resistance loci in segregating populations. Plant J. 2013, 76, 530–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, M.R.; Vossen, J.; Lim, T.Y.; Hutten, R.C.B.; Xu, J.; Strachan, S.M.; Harrower, B.; Champouret, N.; Gilroy, E.M.; Hein, I. Tracking disease resistance deployment in potato breeding by enrichment sequencing. Plant Biotechnol. J. 2018, 17, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.G. The Potato: Evolution, Biodiversity and Genetic Resources; Belhaven Press: London, UK, 1990; p. 259. [Google Scholar]
- Khavkin, E.E.; Fadina, O.A.; Sokolova, E.A.; Beketova, M.P.; Drobyazina, P.E.; Rogozina, E.V.; Kuznetsova, M.A.; Yashina, I.M.; Jones, R.W.; Deahl, K.L. Pyramiding R Genes: Genomic and Genetic Profiles of Interspecific Potato Hybrids and Their Progenitors; PPO-Special Report; Schepers, H.T.A.M., Ed.; DLO Foundation: Wageningen, The Netherlands, 2014; pp. 215–220. [Google Scholar]
- Fadina, O.A.; Beketova, M.P.; Kuznetsova, M.A.; Rogozina, E.V.; Khavkin, E.E. Revisiting Late Blight Resistance Genes in Complex Interspecific Potato Hybrids; PAGV-Special Report; Schepers, H.T.A.M., Ed.; DLO Foundation: Wageningen, The Netherlands, 2017; pp. 245–256. [Google Scholar]
- Yashina, I.M.; Prohorova, O.A.; Kukushkina, L.N. Evaluation of hybrid population of potato for using in breeding on field resistance to late blight. Dostizh. Nauki Tekh. Agroprom. Kompleksa 2010, 12, 17–21. (In Russian) [Google Scholar]
- Rogozina, E.V.; Kolobaev, V.A.; Khavkin, E.E.; Kuznetsova, M.A.; Beketova, M.P.; Sokolova, E.A. Interspecific potato hybrids as a resource for late blight resistance genes. Russ. Agric. Sci. 2014, 40, 10–13. [Google Scholar] [CrossRef]
- Rogozina, E.; Chalaya, N.A.; Kuznetsova, M.A.; Demidova, V.N.; Rogozin, A.N.; Smetanina, T.I.; Beketova, M.P.; Fadina, O.A.; Khavkin, E.E. Late blight resistant potato hybrid clones in the VIR collection of plant genetic resources. Proc. Appl. Bot. Genet. Breed. 2018, 179, 278–292. [Google Scholar] [CrossRef]
- Malcolmson, J.F. Races of Phytophthora infestans occurring in Great Britain. Trans. Br. Mycol. Soc. 1969, 53, 417-IN2. [Google Scholar] [CrossRef]
- Bukasov, S.M. Systematics of the potato. Tr. Prikl. Bot. Genet. Sel. 1978, 62, 3–35. (In Russian) [Google Scholar]
- Spooner, D.M.; Ghislain, M.; Simon, R.; Jansky, S.; Gavrilenko, T.A. Systematics, diversity, genetics, and evolution of wild and cultivated potatoes. Bot. Rev. 2014, 80, 283–383. [Google Scholar] [CrossRef]
- Kuznetsova, M.A.; Yu Spiglazova, S.; Rogozhin, A.N.; Smetanina, T.I.; Filippov, A.V. New Approaches for Measuring Potato Susceptibility to Phytophthora Infestans; PPO-Special Report; Schepers, H.T.A.M., Ed.; DLO Foundation: Wageningen, The Netherlands, 2014; pp. 223–232. [Google Scholar]
- Lapwood, D.H. Laboratory assessments of the susceptibility of potato-tuber tissue to blight (Phytophthora infestans). Potato Res. 1965, 8, 215–229. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-J.; Lee, H.-R.; Jo, K.-R.; Mortazavian, S.M.M.; Huigen, D.J.; Evenhuis, B.; Kessel, G.; Visser, R.G.F.; Jacobsen, E.; Vossen, J.H. Broad spectrum late blight resistance in potato differential set plants MaR8 and MaR9 is conferred by multiple stacked R genes. Theor. Appl. Genet. 2011, 124, 923–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenman, M.; Ali, A.; Mühlenbock, P.; Carlson-Nilsson, U.; Liljeroth, E.; Champouret, N.; Vleeshouwers, V.; Andreasson, E. Effector-driven marker development and cloning of resistance genes against Phytophthora infestans in potato breeding clone SW93-1015. Theor. Appl. Genet. 2015, 129, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Rietman, H.; Bijsterbosch, G.; Cano, L.M.; Lee, H.-R.; Vossen, J.H.; Jacobsen, E.; Visser, R.G.F.; Kamoun, S.; Vleeshouwers, V.G.A.A. Qualitative and quantitative late blight resistance in the potato cultivar sarpo mira is determined by the perception of five distinct RXLR effectors. Mol. Plant-Microbe Interact. 2012, 25, 910–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vossen, J.H.; Van Arkel, G.; Bergervoet, M.; Jo, K.-R.; Jacobsen, E.; Visser, R.G.F. The Solanum demissum R8 late blight resistance gene is an Sw-5 homologue that has been deployed worldwide in late blight resistant varieties. Theor. Appl. Genet. 2016, 129, 1785–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Allefs, S.; Berg, R.G.V.D.; Vleeshouwers, V.; Van Der Vossen, E.A.G.; Vosman, B. Allele mining in Solanum: Conserved homologues of Rpi-blb1 are identified in Solanum stoloniferum. Theor. Appl. Genet. 2008, 116, 933–943. [Google Scholar] [CrossRef]
- Colton, L.M.; Groza, H.I.; Wielgus, S.M.; Jiang, J. Marker-Assisted selection for the broad-spectrum potato late blight resistance conferred by gene rb derived from a wild potato species. Crop. Sci. 2006, 46, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Vossen, E.A.G.V.D.; Gros, J.; Sikkema, A.; Muskens, M.; Wouters, D.; Wolters, P.; Pereira, A.; Allefs, S. The Rpi-blb2 gene from Solanum bulbocastanum is an Mi-1 gene homolog conferring broad-spectrum late blight resistance in potato. Plant J. 2005, 44, 208–222. [Google Scholar] [CrossRef]
- Pel, M.A. Mapping, Isolation and Characterization of Genes Responsible for Late Blight Resistance in Potato. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2010; p. 209. [Google Scholar]
- Chávez, R.S.; Sosa, M.H. Use of dihaploids in the breeding of Solanum tuberosum L. (I. Cytological considerations). Hereditas 2009, 69, 83–99. [Google Scholar] [CrossRef]
- Aguilera-Galvez, C.; Champouret, N.; Rietman, H.; Lin, X.; Wouters, D.; Chu, Z.; Jones, J.; Vossen, J.; Visser, R.; Wolters, P.J.; et al. Two different R gene loci co-evolved with Avr2 of Phytophthora infestans and confer distinct resistance specificities in potato. Stud. Mycol. 2018, 89, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.A.; Fadina, O.A.; Khavkin, E.E.; Rogozina, E.V.; Kuznetsova, M.A.; Jones, R.W.; Deahl, K.L. Structural Homologues of CC-NBS-LRR Genes for Potato Late Blight Resistance in Wild Solanum Species; PPO-Special Report; Schepers, H.T.A.M., Ed.; DLO Foundation: Wageningen, The Netherlands, 2014; pp. 247–253. [Google Scholar]
- Fadina, O.A.; Beketova, M.P.; Kuznetsova, M.A.; Rogozina, E.V.; Khavkin, E.E. Polymorphisms and evolution of Solanum bulbocastanum genes for broad-spectrum resistance to Phytophthora infestans. Russ. J. Plant Physiol. 2019, 66, 950–957. [Google Scholar] [CrossRef]
- Fadina, O.A.M.; Beketova, M.P.; Kuznetsova, M.A.; Rogozina, E.V.; Khavkin, E.E. South American species Solanum alandiae Card. and S. okadae Hawkes et Hjerting as potential sources of genes for potato late blight resistance. Proc. Appl. Bot. Genet. Breed. 2020, 181, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, D.; Xu, Y.; Zhao, H.; Wang, L.; Cao, X.; Chen, Y.; Chen, Q. A new resistance gene against potato late blight originating from Solanum pinnatisectum located on potato chromosome 7. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Li, J.; Tian, Z.; Du, J.; Armstrong, M.; Baker, K.; Lim, T.Y.; Vossen, J.H.; He, H.; Portal, L.; et al. Potato late blight field resistance from QTL dPI09c is conferred by the NB-LRR gene R8. J. Exp. Bot. 2018, 69, 1545–1555. [Google Scholar] [CrossRef]
- Beketova, M.P.; Sokolova, E.A.; Rogozina, E.V.; Kuznetsova, M.A.; Khavkin, E.E. Two orthologs of late blight resistance gene R1 in wild and cultivated potato. Russ. J. Plant Physiol. 2017, 64, 718–727. [Google Scholar] [CrossRef]
- Van Lieshout, N.; Van Der Burgt, A.; De Vries, M.E.; Ter Maat, M.; Eickholt, D.; Esselink, D.; Van Kaauwen, M.P.W.; Kodde, L.P.; Visser, R.G.F.; Lindhout, P.; et al. Solyntus, the new highly contiguous reference genome for potato (Solanum tuberosum). G3 Genes Gen. Genet. 2020, 10, 3489–3495. [Google Scholar] [CrossRef]
- Vleeshouwers, V.; Raffaele, S.; Vossen, J.H.; Champouret, N.; Oliva, R.; Segretin, M.E.; Rietman, H.; Cano, L.M.; Lokossou, A.; Kessel, G.; et al. Understanding and exploiting late blight resistance in the age of effectors. Ann. Rev. Phytopathol. 2011, 49, 507–531. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, R.A. Pre-emptive breeding to control wheat rusts. Euphytica 1992, 63, 103–113. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Brown, G.N. Anticipatory breeding for resistance to rust diseases in wheat. Ann. Rev. Phytopathol. 1997, 35, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Raffaele, S.; Kamoun, S. The two-speed genomes of filamentous pathogens: Waltz with plants. Curr. Opin. Genet. Dev. 2015, 35, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Thilliez, G.; Armstrong, M.R.; Lim, T.; Baker, K.; Jouet, A.; Ward, B.; Van Oosterhout, C.; Jones, J.D.G.; Huitema, E.; Birch, P.R.; et al. Pathogen enrichment sequencing (PenSeq) enables population genomic studies in oomycetes. N. Phytol. 2019, 221, 1634–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, B.A.; Stukenbrock, E.H. Rapid emergence of pathogens in agro-ecosystems: Global threats to agricultural sustainability and food security. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Series in the Section Petota | Species | Countries | Germplasm Codes |
|---|---|---|---|
| Acaulia Juz. | S. acaule Bitt. | Argentina, Bolivia, Peru | acl |
| Bulbocastana (Rydb.) Hawkes | S. bulbocastanum Dun. | Guatemala, Mexico | blb |
| Commersoniana Buk. | S. commersonii Dun. | Argentina, Brazil, Paraguay, Uruguay | cmm |
| Demissa Buk. | S. demissum Lindl. | Mexico, Guatemala | dms |
| S. × edinense Berth. | Mexico | edn | |
| S. × semidemissum Juz. | Mexico | sem | |
| Longipedicellata Buk. | S. antipoviczii Buk. = S. stoloniferum | Mexico | ant |
| S. polytrichon Rydb. = S. stoloniferum | Mexico | plt | |
| S. stoloniferum Schlechtd. & Bché. | Mexico | sto | |
| S. ×vallis-mexici Juz. | Mexico | vll | |
| Megistacroloba Cárdenas & Hawkes | S. megistacrolobum Bitt. | Peru, Bolivia, Argentina | mga |
| Pinnatisecta (Rydb.) Hawkes | S. pinnatisectum Dun. | Mexico | pnt |
| Tuberosa (Rydb.) Hawkes | S. alandiae Cárd. | Bolivia | aln |
| S. andigenum Juz. & Buk. = S. tuberosum ssp. andigena Hawkes | Argentina, Bolivia, Guatemala, Colombia, Ecuador, Mexico, Peru, Venezuela | adg | |
| S. berthaultii Hawkes | Bolivia | ber | |
| S. brevicaule Bitt. | Bolivia | brc | |
| S. chilotanum (Buk. & Lechn.) Hawkes (= S. tuberosum ssp. tuberosum L.). | Chile | chi | |
| S. leptostigma Juz. (= S. tuberosum ssp. tuberosum L.). | Chile | lpt | |
| S. microdontum Bitt. | Argentina, Bolivia | mcd | |
| S. okadae Hawkes & Hjert. | Argentina, Bolivia | oka | |
| S. phureja Juz. & Buk. | Ecuador, Colombia, Venezuela, Bolivia, Peru | phu | |
| S. rybinii Juz. & Buk. (=S. phureja Juz. & Buk.) | Ecuador, Colombia, Venezuela, Bolivia, Peru | ryb | |
| S. simplicifolium Bitt. = S. microdontum | Argentina, Bolivia | sim | |
| S. spegazzinii Bitt. | Argentina | spg | |
| S. vernei Bitt. & Wittm. | Argentina | vrn | |
| S. verrucosum Schlechtd. | Mexico | ver | |
| Yungasensa Corr. | S. chacoense Bitt. | Argentina, Bolivia, Brazil, Paraguay, Uruguay | chc |
| Hybrid, Cultivar * | Bred from | Pedigree **** | LB Resistance ***** | ||
|---|---|---|---|---|---|
| ♀ Female | ♂ Male | Field | Laboratory | ||
| Hybrids bred by I.M. Yashina | |||||
| 2585-67 | F1 | Nikulinskij {Mavka × [Apta (Interspecific hybrid × Hindenburg)] × Karpatskij} | Peterburgskij [(Omega (adg, dms, chi) × E 109/11]× | 7 | 6 |
| 2585-70 | F1 | Nikulinskij | Peterburgskij | 5 | 4 |
| 2585-80 | F1 | Nikulinskij | Peterburgskij | 7 | 6 |
| 97.12-18 | F1 | Nikulinskij | 88.16/20 {[(S.chacoense × S. tuberosum) × Kameraz]} × Belorusskij 3} | 5 | 4 |
| 2359-13 | F1 | Nikulinskij | 88.16/20 {[(S.chacoense × S. tuberosum) × Kameraz]} × Belorusskij 3} | 6 | 5 |
| 2584-7 | F1 | Nikulinskij | Ausonia (Wilja × Konst 63-655 adg) | 6 | 4 |
| 97.13-9 | F1 | Nikulinskij | 375.333.1 (cmm, dms, mga) | 5 | 3 |
| 97.1.17 | F1 | Lugovskoj (164-1С/72 × 60С/73) | 88.16/20 {[(S.chacoense × S. tuberosum) × Kameraz]} × Belorusskij 3} | 7 | 4 |
| 2372-60 | F1 | 1977-76 | Zarevo (7692 С 68 × Bekra) adg, dms, plt | 8 | 6 |
| 2522-173 | F1 | Utenok {Adretta × [(Saskia × Ora) × [(Apta × MPI 44335 1309 (adg,dms)) × Schwalbe] Lu.59.884/3 × Axilia] × 15-26 [Lyubimec × 172m-7 (S.chacoense×S. tuberosum)]} | 90/2 | 6 | 3 |
| Hybrids bred by V.A. Kolobaev | |||||
| 10/5-09 | F1 | Zagadka Pitera (dms, phu, sto, tbr, vrn) | mixture of pollen *** | 6–7 | 4 |
| 11/6-09 | F2 | Zagadka Pitera (dms, phu, sto, tbr, vrn) | mixture of pollen | 6–7 | 4 |
| 12/1-09 | F4 | S. pinnatisectum k-17464 | Fausta (Sommerstarke (dms) × W8102/214) | 6–7 | 6 |
| 13/11-09 | F1 | F2 (S. pinnatisectum k-17464 × Gitte (adg)) | mixture of pollen | 7 | 5 |
| 14/8-09 | F5 | (S. polytrichon k-5345 × MPI 50-140\5 (ant = sto, dms)) | MPI 50-140\5 (ant = sto, dms) | 6 | 4 |
| 15/13-09 | F1 | (S. pinnatisectum k-17464 × Gitte (adg)) | F2 [(S. polytrichon k-5345 × MPI 50-140/5 (ant=sto)) × MPI 50-140/5] × |F3[(S. verrucosum × MPI 50-140/5) × Licaria] × F2 ⁅F2[(S. polytrichon k-5345 × MPI 50-140/5) × MPI 50-140/5] × {[(S. simplicifolium k-5400 × MPI 50-140/5) × Mariella (adg, dms)] × Desiree}⁆| | 6 | 6 |
| 16/27-09 | F1 | [(S. berthaultii k-8510 × Tajga (adg, dms)) × Оmega (adg, chi, dms)] × F2[(S. polytrichon k-5345 × MPI 50-140/5 (ant=sto) × MPI 50-140/5] × F2{[(S. simplicifolium k-5400 × MPI 50-140/5) × Gitte (adg)] × Hera}⁆| | Nayada (adg, dms, phu, sto, tbr, vrn) | 7 | 6 |
| 18/40-2000 | F2 | [(S. polytrichon k-5345 × MPI 50-140/5 (ant=sto)) × Umbra] × Fausta (dms) | [(S. simplicifolium k-5400 × MPI 50-140/5) × Gitte (adg)] × Hera | 6 | 5 |
| 111 (38 KVA) | F1 | Fermer | F4⁅F2[(S. polytrichon k-5345 × MPI 50-140/5 (ant=sto)) × MPI 50-140/5] × F2{[(S. simplicifolium k-5400 × MPI 50-140/5) × Gitte (adg)] × Hera}⁆ | 6.5–8 | 6 |
| 113 (50/1 KVA) | F1 | Zagadka Pitera (dms, phu, sto, tbr, vrn) × mixture of pollen | Nayada (adg, dms, phu, sto, tbr, vrn) × mixture of pollen | 6–7 | 6 |
| Hybrids bred by E.V. Rogozina | |||||
| 117-1 | F1 | Atzimba (adg, dms) | S. alandiae k-21240 | ||
| 117-2 | F1 | Atzimba | S. alandiae k-21240 | 5–7 | 5 |
| 39-1-2005 | F1 | Atzimba | S. alandiae k-21240 | 6–7 | 6 |
| 24-1 | F1 | Atzimba | S. alandiae k-21240 | 6–8 | 7 |
| 24-2 | F1 | Atzimba | S. alandiae k-21240 | 6–8 | 7 |
| 25-1-2007 | F1 | Elizaveta | 24-1 (Atzimba × S. alandiae k-21240) | 5 | 5 |
| 25-2-2007 | F1 | Elizaveta | 24-1 (Atzimba × S. alandiae k-21240) | 4–5 | 4 |
| 134-2-2006 | F1 | 24-2 (Atzimba × S. alandiae k-21240) | Svitanok kievskij | 6–7 | 6 |
| 134-3-2006 | F1 | 24-2 (Atzimba × S. alandiae k-21240) | Svitanok kievskij | 2–3 | 3 |
| 134-6-2006 | F1 | 24-2 (Atzimba × S. alandiae k-21240) | Svitanok kievskij | 5–6 | 5 |
| 135-1-2006 | F1 | Svitanok kievskij | 24-2 (Atzimba × S. alandiae k-21240) | 5–7 | 5 |
| 135-2-2006 | F1 | Svitanok kievskij | 24-2 (Atzimba × S. alandiae k-21240) | 4.5–7 | 4 |
| 139 (4-1-2012) | F1 | Atzimba × S. alandiae k-21240 | F5 [(S. polytrichon k-5345 × MPI 50-140\5) × MPI 50-140\5] | 7–9 | 6 |
| 97-155-1 | F1 | Bobr (adg, dms, sto) | 91-21-4 (adg, dms, ryb) | 7–8 | 6 |
| 128-05-03 | F1 | 97-155-1 (adg, ryb, sto) | Nayada (adg, dms, phu, sto, tbr, vrn) | 6–7 | 5 |
| 118 (118-5-2011) | F2 | Bobr (adg, dms, sto) | 91-21-4 (adg, dms, ryb) | 5–8 | 6 |
| 120 (118-6-2011) | F2 | Bobr (adg, dms, sto) | 91-21-4 (adg, dms, ryb) | 5–7 | 5 |
| 160-1 | F2 | Bobr (adg, dms, sto) | 91-21-4 (adg, dms, ryb) | 7–8 | nd |
| 160-17 | F2 | Bobr (adg, dms, sto) | 91-21-4 (adg, dms, ryb) | 6–7 | 5 |
| 106 (171-3) | F2 | Bobr (adg, dms, sto) | 91-21-4 (adg, dms, ryb) | 6–7 | 6 |
| 123 (128-6) | F2 | Bobr (adg, dms, sto) | 91-21-4 (adg, dms, ryb) | 6–8 | 6 |
| 90-6-2 | F1 | 194-4 (adg, phu, sto) | CIP-1039 (adg) | 7 | nd |
| 99-6-5 | F1 | 90-6-2 (adg, phu, sto) | Hertha (adg, dms, ryb, tbr) | 3–4 | nd |
| 99-6-6 | F1 | 90-6-2 (adg, phu, sto) | Hertha (adg, dms, ryb, tbr) | 5 | nd |
| 97-153-2 | F1 | 90-6-2 (adg, phu, sto) | 91-21-4 (adg, dms, ryb) | 6 | 5 |
| 2 (194-4т) | F1 | Zagadka Pitera (dms, sto, vrn, phu, tbr) | 99-6-6 (adg, dms, phu, ryb, sto, tbr) | 6–7 | 5 |
| 99-4-1 | F1 | 180-1 (sto) | Hertha (adg, dms, ryb, tbr) | 5–7 | 5 |
| 7 (93-5-30) | F1 | 41.85.6 (adg, phu, ryb) | 91-19-2 (acl, blb, sto) | 5–7 | 5 |
| 190-4 | F1 | Gibridnyj 14 (dms, vll) | 194-4 (adg, phu, sto) | 7–8 | 4 |
| 97-162-2 | F1 | 91-15-2 (adg, ryb, sto) | 90-21-1 (adg, mcd, ryb, spg, sto) | 3 | nd |
| 34-6 | F1 | 97-162-2 (adg, mcd, ryb, spg, sto) | 190-4 (adg, dms, phu, sto, vll) | 5 | nd |
| 53 (34-5-2003) | F1 | 97-162-2(adg, mcd, ryb, spg, sto) | 190-4 (adg, dms, phu, sto, vll) | 6 | 5 |
| 135-3-2005 | F1 | S. okadae k-20921 | S. chacoense k-19759 | 5 | nd |
| 135-5-2005 | F1 | S. okadae k-20921 | S. chacoense k-19759 | 5 | nd |
| 8-1-2004 | F1 | S. okadae k-20921 | S. chacoense k-19759 | 5 | nd |
| 8-3-2004 | F1 | S. okadae k-20921 | S. chacoense k-19759 | 3 | nd |
| 8-5-2004 | F1 | S. okadae k-20921 | S. chacoense k-19759 | 5 | nd |
| Other hybrids and cultivars employed as standards | |||||
| R5 | nd ** | nd | nd | ||
| R8 | nd | nd | nd | ||
| R9 | nd | nd | nd | ||
| Magellanes | nd | indigenous cultivar of Chile S. tuberosum ssp. tuberosum L. | - | nd | nd |
| Alouette | nd | AR 02-139-1 | Laura | 8–9 | 7 |
| Atzimba | F1 | US 133.3 | 52- AT-1 (adg) | 5 | 4 |
| Sarpo Axona | nd | nd | nd | 8 | 7 |
| Sarpo Mira | 76 PO 12 14 268 | D 187 | 8 | 7 | |
| Alpha | F1 | Paul Kruger | Preferent | 4 | 3 |
| Bintje | F1 | Munstersen | Fransen | 3 | 3 |
| Desiree | F1 | Urgenta | Depesche | 4 | 2 |
| Early Rose | nd | Garnet Chili | - | 4 | nd |
| Eersteling | nd | Duke of York | - | 4 | 3 |
| Escort | F1 | Rental | Cebeco 64 197 16 (dms) | 6–7 | 6 |
| Gloria | nd | Alpha? | Bato | 5 | 3–4 |
| Jubel | F1 | Victoria Augusta | 78 92 | 7 | nd |
| Robijn | nd | Rode Star | Preferent | 5 | 4 |
| Elizaveta | F1 | acl, adg, dms, phu, sto, tbr, vrn | nd | 5 | 4 |
| Nayada | nd | adg, dms, phu, sto, tbr, vrn | nd | 6 | 5 |
| Negr | nd | indigenous cultivar of Chile S. tuberosum ssp. tuberosum L. | - | 4 | 3 |
| Priekul’skij rannij | nd | Irish Cobbler | Jubel | 5 | 3 |
| Svitanok kievskij | nd | Adretta (adg, dms) | 3774c 71 | 5 | 4 |
| Zagadka Pitera | nd | dms, phu, sto, tbr, vrn | nd | 6 | 5 |
| Gene | Prototype Gene * | Marker, Size, bp. | Position on the Gene, bp | Primers Sequences | Anneal. Temp., °C | References |
|---|---|---|---|---|---|---|
| Rpi-R1 | AF447489 | Rpi-R1-1205 | 5126–6331 | F-cactcgtgacatatcctcacta R-gtagtacctatcttatttctgcaagaat | 61 | [21] |
| Rpi-R2/Rpi-blb3 | FJ536325 | Rpi-R2-686 | 1370–2055 | F-gctcctgatacgatccatg R-acggcttcttgaatgaa | 54 | [38] |
| Rpi-R2-1137 | 1277–2413 | F-aagatcaagtggtaaaggctgatg R-atctttctagcttccaaagatcacg | 60 | [39] | ||
| FJ536346 | Rpi-blb3-305 | 5551–5855 | F–agctttttgagtgtgtaattgg R-gtaactacggactcgaggg | 63.5 | [8] | |
| Rpi-R3a | AY849382 | Rpi-R3a-1380 | 1677–3056 | F-gtagtacctatcttatttctgcaagaat R-agccacttcagcttcttacagtagg | 64 | [21] |
| Rpi-R3b | JF900492 | Rpi-R3b-378 | 94818–95195 | F-gtcgatgaatgctatgtttctcgaga R-accagtttcttgcaattccagattg’ | 64 | [40] |
| Rpi-R8 | KU530153 | Rpi-R8-1276 | 73694–74970 | F-aacaagagatgaattaagtcggtagc R-gctgtaggtgcaatgttgaagga | 62.5 | [41] modif. |
| Rpi-blb1 = Rpi-sto1 | AY336128 | Rpi-blb1-821 | 2304–3124 | F-aacctgtatggcagtggcatg R-gtcagaaaagggcactcgtg | 62 | [42] |
| AY336128 | Rpi-blb1-226 | 3143–3368 | F–cacgaggcccttttctgac R-ttcaattgtgttgcgcactag | 50 | [43] | |
| EU884421 | Rpi-sto1-890 | 241–1130 | F-accaaggccacaagattctc R-cctgcggttcggttaataca | 65 | [8] | |
| Rpi-blb2 | DQ122125 | Rpi-blb2-976 | 3226–4202 | F-ggactgggtaacgacaatcc R-atttatggctgcagaggacc | 55 | [44] |
| Rpi-vnt1 | FJ423046 | Rpi-vnt1.3-612 | 89–701 | F-ccttcctcatcctcacatttag R-gcatgccaactattgaaacaac | 58 | [45] |
| Geno-Types | Solanum Species in Hybrid Pedigrees * | Genes | The Number of Genes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 = Rpi-blb3 | R3a | R3b | R8 | Rpi-blb 1 = Rpi-sto1 | Rpi-blb2 | Rpi-vnt1-3 | |||||||
| R1-1205 | R2-1137 | R2-686 | Rpi-blb3-305 | R3a-1380 | R3b-378 | R8-1276 | RB-226 | Rpi-blb1-821 | Rpi-sto1-890 | Rpi-blb2-976 | Rpi-vnt1.3-612 | |||
| Hybrids bred by I.M. Yashina | ||||||||||||||
| 2585-67 | adg, chi, dms, tbr | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| 2585-70 | adg, chi, dms, tbr | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| 2585-80 | adg, chi, dms, tbr | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 2359-13 | chc, dms, tbr | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| 2584-7 | adg, chc, dms, edn, ryb, tbr | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 97.12-18 | chc, dms, tbr | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| 97.13-9 | cmm, dms, mga, tbr | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 5 |
| 2372-60 | adg, chc, dms, lpt, sto, tbr, | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| 2522-173 | adg, chc, dms, tbr | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| 97.1.17 | adg, chc, dms, sem, tbr | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 10/5-09 | dms, phu, sto, tbr, vrn | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 5 |
| 11/6-09 | dms, phu, sto, tbr, vrn | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 5 |
| 12/1-09 | dms, pnt, tbr | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 |
| 13/11-09 | adg, pnt, tbr | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 4 |
| 14/8-09 | Ant = sto, dms, plt = sto, tbr | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 5 |
| 15/13-09 | adg, ant = sto, dms, plt = sto, pnt, sim = mcd, tbr, ver | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 5 |
| 16/27-09 | adg, ant = sto, ber, chi, dms, phu, plt = sto, sim = mcd, tbr, vrn | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 4 |
| 18/40-2000 | adg, dms, mcd, plt = sto, sto, tbr, | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 3 |
| 111 (38 KVA) | adg, ant = sto, dms, plt = sto, sim = mcd, tbr | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 5 |
| 113 (50/1 KVA) | adg, dms, phu, sto, tbr, vrn | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 4 |
| Hybrids bred by E.V. Rogozina | ||||||||||||||
| 117-1 | adg, aln = brc, dms, tbr | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| 117-2 | adg, aln = brc, dms, tbr | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| 39-1-2005 | adg, aln = brc, dms, tbr | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 3 |
| 24-1 | adg, aln = brc, dms, tbr | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 4 |
| 24-2 | adg, aln = brc, dms, tbr | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 4 |
| 25-1-2007 | acl, adg, aln = brc, dms, phu, sto, tbr, vrn | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 3 |
| 25-2-2007 | acl, adg, aln = brc, dms, phu, sto, tbr, vrn | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| 134-2-2006 | adg, aln = brc, dms, tbr | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 5 |
| 134-3-2006 | adg, aln = brc, dms, tbr | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| 134-6-2006 | adg, aln = brc, dms, tbr | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 4 |
| 135-1-2006 | adg, aln = brc, dms, tbr | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 4 |
| 135-2-2006 | adg, aln = brc, dms, tbr | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 5 |
| 139 (4-1-2012) | adg, aln = brc, ant = sto, dms, plt = sto, tbr | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 3 |
| 97-155-1 | adg, dms, ryb, sto, tbr | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 5 |
| 128-05-03 | adg, dms, phu, ryb, sto, tbr, vrn | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 4 |
| 118 (118-5-2011) | adg, dms, ryb, sto, tbr | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 4 |
| 120 (118-6-2011) | adg, dms, ryb, sto, tbr | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 4 |
| 160-1 | adg, dms, ryb, sto, tbr | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| 160-17 | adg, dms, ryb, sto, tbr | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| 106 (171-3) | adg, dms, ryb, sto, tbr | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| 123 (128-6) | adg, dms, ryb, sto, tbr | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 5 |
| 90-6-2 | adg, phu, sto, tbr | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 5 |
| 99-6-5 | adg, dms, phu, sto, tbr | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 5 |
| 99-6-6 | adg, dms, phu, sto, tbr | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 6 |
| 97-153-2 | adg, dms, phu, sto, tbr | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| 2 (194-4т) | adg, dms, phu, ryb, sto, tbr, vrn | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 3 |
| 99-4-1 | adg, dms, ryb, sto, tbr | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 7 (93-5-30) | acl, adg, blb, dms, phu, ryb, sto, tbr | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 3 |
| 190-4 | adg, dms, phu, sto, tbr, vll | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 5 |
| 97-162-2 | adg, mcd, ryb, spg=brc, sto, tbr | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| 34-6 | adg, mcd, ryb, spg=brc, sto, phu, tbr, vll | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 |
| 53 (34-5-2003) | adg, mcd, ryb, spg=brc, sto, phu, tbr, vll | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| 135-3-2005 | chc, oka | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 |
| 135-5-2005 | chc, oka | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| 8-1-2004 | chc, oka | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 8-3-2004 | chc, oka | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| 8-5-2004 | chc, oka | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Reference genotypes | ||||||||||||||
| R5 | dms, tbr | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 5 |
| R8 | dms, tbr | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 4 |
| R9 | dms, tbr | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 5 |
| Magel-lanes | S. tuberosum ssp. tuberosum L. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| Alouette | vnt | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 3 |
| Atzimba | adg, dms, tbr | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 3 |
| Sapro Axona | dms, tbr | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Sapro Mira | dms, tbr | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| Alpha | tbr | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Bintje | tbr | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Desiree | tbr | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Early Rose | tbr | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| Eersteling | tbr | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Escort | dms, tbr | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Gloria | adg, dms, tbr | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Jubel | dms?, tbr | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| Robijn | tbr | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Elizaveta | acl, adg, dms, phu, sto, tbr, vrn | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 4 |
| Nayada | adg, dms, phu, sto, tbr, vrn | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 4 |
| Negr | S. tuberosum ssp. tuberosum L. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Priekul’skij rannij | tbr | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 |
| Svitanok kievskij | dms, tbr | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 5 |
| Zagadka Pitera | dms, phu, sto, tbr, vrn | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 4 |
| Genotype | Pedigree | Rpi-R1 | Rpi-R2/Rpi-blb3 | Rpi-R3a | Rpi-R3b | Rpi-R8 | Rpi-blb1/Rpi-sto1 | Rpi-blb2 | Rpi-vnt1 | Total Gene Number | Field Resistance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2359-13 | chc, dms, tbr | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 4 | 6 |
| 97.13-9 | cmm, dms, mga, tbr | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 5 | 5 |
| 2372-60 | adg, chc, dms, lpt, sto, tbr | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 4 | 8 |
| 10/5-09 | dms, phu, sto, tbr, vrn | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 5 | 7 |
| 11/6-09 | dms, phu, sto, tbr, vrn | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 5 | 7 |
| 13/11-09 | adg, pnt, tbr | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 4 | 7 |
| 14/8-09 | Ant = sto, dms, plt = sto, tbr | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 5 | 6 |
| 15/13-09 | adg, ant = sto, dms, plt = sto, pnt, sim = mcd, tbr, ver | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 5 | 6 |
| 16/27-09 | adg, ant = sto, ber, chi, dms, phu, plt = sto, sim = mcd, tbr, vrn | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 4 | 7 |
| 111 (38 KVA) | adg, ant = sto, dms, plt = sto, sim = mcd, tbr | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 5 | 8 |
| 113 (50/1 KVA) | adg, dms, phu, sto, tbr, vrn | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 4 | 7 |
| 117-2 | adg, aln = brc, dms, tbr | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 4 | 7 |
| 24-1 | adg, aln = brc, dms, tbr | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 4 | 8 |
| 24-2 | adg, aln = brc, dms, tbr | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 4 | 8 |
| 25-2-2007 | acl, adg, aln = brc, dms, phu, sto, tbr, vrn | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 5 | 5 |
| 134-2-2006 | adg, aln = brc, dms, tbr | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 5 | 7 |
| 134-6-2006 | adg, aln = brc, dms, tbr | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 4 | 6 |
| 135-1-2006 | adg, aln = brc, dms, tbr | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 4 | 7 |
| 135-2-2006 | adg, aln = brc, dms, tbr | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 4 | 7 |
| 97-155-1 | adg, dms, ryb, sto, tbr | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 5 | 8 |
| 128-05-03 | adg, dms, phu, ryb, sto, tbr, vrn | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 4 | 7 |
| 118 (118-5-2011) | adg, dms, ryb, sto, tbr | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 4 | 8 |
| 120 (118-6-2011) | adg, dms, ryb, sto, tbr | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 4 | 7 |
| 123 (128-6) | adg, dms, ryb, sto, tbr | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 5 | 8 |
| 90-6-2 | adg, phu, sto, tbr | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 5 | 7 |
| 99-6-5 | adg, phu, sto, tbr | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 5 | 4 |
| 99-6-6 | adg, phu, sto, tbr | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 | 5 |
| 190-4 | adg, dms, phu, sto, tbr, vll | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 5 | 8 |
| Escort | dms, tbr | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 4 | 7 |
| Jubel | dms?, tbr | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 5 | 7 |
| Elizaveta | acl, adg, dms, phu, sto, tbr, vrn | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 4 | 5 |
| Nayada | adg, dms, phu, sto, tbr, vrn | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 4 | 6 |
| Svitanok kievskij | dms, tbr | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 5 | 5 |
| Zagadka Pitera | dms, phu, sto, tbr, vrn | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 4 | 6 |
| Frequency | 0.44 | 0.74 | 0.59 | 0.79 | 0.66 | 0.41 | 0.44 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogozina, E.V.; Beketova, M.P.; Muratova, O.A.; Kuznetsova, M.A.; Khavkin, E.E. Stacking Resistance Genes in Multiparental Interspecific Potato Hybrids to Anticipate Late Blight Outbreaks. Agronomy 2021, 11, 115. https://doi.org/10.3390/agronomy11010115
Rogozina EV, Beketova MP, Muratova OA, Kuznetsova MA, Khavkin EE. Stacking Resistance Genes in Multiparental Interspecific Potato Hybrids to Anticipate Late Blight Outbreaks. Agronomy. 2021; 11(1):115. https://doi.org/10.3390/agronomy11010115
Chicago/Turabian StyleRogozina, Elena V., Mariya P. Beketova, Oksana A. Muratova, Mariya A. Kuznetsova, and Emil E. Khavkin. 2021. "Stacking Resistance Genes in Multiparental Interspecific Potato Hybrids to Anticipate Late Blight Outbreaks" Agronomy 11, no. 1: 115. https://doi.org/10.3390/agronomy11010115
APA StyleRogozina, E. V., Beketova, M. P., Muratova, O. A., Kuznetsova, M. A., & Khavkin, E. E. (2021). Stacking Resistance Genes in Multiparental Interspecific Potato Hybrids to Anticipate Late Blight Outbreaks. Agronomy, 11(1), 115. https://doi.org/10.3390/agronomy11010115