Agronomic Biofortification of Significant Cereal Crops with Selenium—A Review
Abstract
:1. Introduction
2. Selenium in the Environment
2.1. Selenium in Soils
2.2. Selenium in Plants
2.3. The Impact of Selenium Bioavailability on Human Health
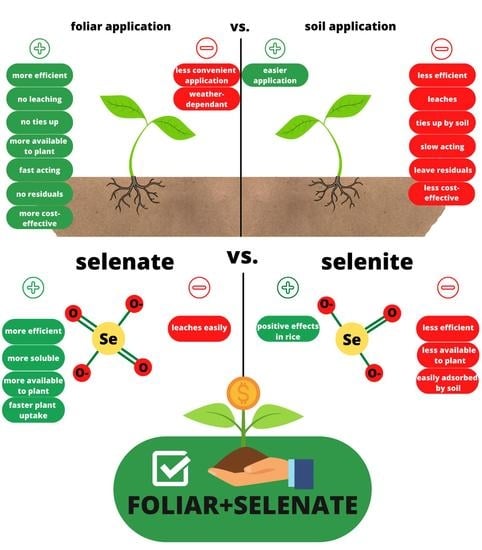
3. Agronomic Biofortification
4. Selenium Content Affected by the Method and Form of Selenium: Meta-Analysis
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Irshad, S.; Abbas, Q.; Ahmad, R. A Comprehensive Review on Environmental Transformation of Selenium: Recent Advances and Research Perspectives; Springer: Dordrecht, The Netherlands, 2019; Volume 41, ISBN 0123456789. [Google Scholar]
- Terry, N.; Zayed, A.M.; De Souza, M.P.; Tarun, A.S. Selenium in Higher Plants. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [Green Version]
- El-Ramady, H.; Abdalla, N.; Alshaal, T.; Domokos-Szabolcsy, É.; Elhawat, N.; Prokisch, J.; Sztrik, A.; Fári, M.; El-Marsafawy, S.; Shams, M.S. Selenium in soils under climate change, implication for human health. Environ. Chem. Lett. 2014, 13, 1–19. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Al Mahmud, J.; Nahar, K.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- dos Reis, A.R.; El-Ramady, H.; Santos, E.F.; Gratão, P.L.; Schomburg, L. Overview of Selenium Deficiency and Toxicity Worldwide: Affected Areas, Selenium-Related Health Issues, and Case Studies. In Selenium in Plants; Springer: Cham, Switzerland, 2017; pp. 209–230. [Google Scholar] [CrossRef]
- Finley, J.W. Selenium accumulation in plant foods. Nutr. Rev. 2005, 63, 196–202. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Faizy, S.E.D.; Abdalla, N.; Taha, H.; Domokos-Szabolcsy, É.; Fari, M.; Elsakhawy, T.; Omara, A.E.D.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and nano-selenium biofortification for human health: Opportunities and challenges. Soil Syst. 2020, 4, 57. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H. On the ecology of selenium accumulation in plants. Plants 2019, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Schiavon, M.; Berto, C.; Malagoli, M.; Trentin, A.; Sambo, P.; Dall’Acqua, S.; Pilon-Smits, E.A.H. Selenium biofortification in radish enhances nutritional quality via accumulation of methyl-selenocysteine and promotion of transcripts and metabolites related to glucosinolates, phenolics amino acids. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Ríos, J.J.; Rosales, M.A.; Blasco, B.; Cervilla, L.M.; Romero, L.; Ruiz, J.M. Biofortification of Se and induction of the antioxidant capacity in lettuce plants. Sci. Hortic. 2008, 116, 248–255. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadley, M.R.; Alcock, J.; Alford, J.; Cartwright, P.; Foot, I.; Fairweather-Tait, S.J.; Hart, D.J.; Hurst, R.; Knott, P.; McGrath, S.P.; et al. Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant. Soil 2010, 332, 5–18. [Google Scholar] [CrossRef]
- Sarwar, H. The importance of cereals (Poaceae: Gramineae) nutrition in human health: A review. J. Cereal. Oilseeds 2013, 4, 32–35. [Google Scholar] [CrossRef]
- Bocchini, M.; D’Amato, R.; Ciancaleoni, S.; Fontanella, M.C.; Palmerini, C.A.; Beone, G.M.; Onofri, A.; Negri, V.; Marconi, G.; Albertini, E.; et al. Soil selenium (Se) biofortification changes the physiological, biochemical and epigenetic responses to water stress in Zea mays L. by inducing a higher drought tolerance. Front. Plant Sci. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Abenavoli, L.; Milanovic, M.; Procopio, A.C.; Spampinato, G.; Maruca, G.; Perrino, E.V.; Mannino, G.C.; Fagoonee, S.; Luzza, F.; Musarella, C.M. Ancient wheats: Beneficial effects on insulin resistance. Minerva Med. 2021. [Google Scholar] [CrossRef]
- Narwal, S.; Kumar, D.; Kharub, A.S.; Verma, R.P.S. Barley Biofortification: Present Status and Future Prospects; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128184448. [Google Scholar]
- Premarathna, L.; McLaughlin, M.J.; Kirby, J.K.; Hettiarachchi, G.M.; Stacey, S.; Chittleborough, D.J. Selenate-enriched urea granules are a highly effective fertilizer for selenium biofortification of paddy rice grain. J. Agric. Food Chem. 2012, 60, 6037–6044. [Google Scholar] [CrossRef] [Green Version]
- De Vita, P.; Platani, C.; Fragasso, M.; Ficco, D.B.M.; Colecchia, S.A.; Del Nobile, M.A.; Padalino, L.; Di Gennaro, S.; Petrozza, A. Selenium-enriched durum wheat improves the nutritional profile of pasta without altering its organoleptic properties. Food Chem. 2017, 214, 374–382. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, Z.; Shui, Y.; Liu, X.; Chen, J.; Khan, S.; Wang, J.; Gao, Z. Methods of Selenium Application Differentially Modulate Plant Growth, Selenium Accumulation and Speciation, Protein, Anthocyanins and Concentrations of Mineral Elements in Purple-Grained Wheat. Front. Plant Sci. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Zou, C.; Du, Y.; Rashid, A.; Ram, H.; Savasli, E.; Pieterse, P.J.; Ortiz-Monasterio, I.; Yazici, A.; Kaur, C.; Mahmood, K.; et al. Simultaneous Biofortification of Wheat with Zinc, Iodine, Selenium, and Iron through Foliar Treatment of a Micronutrient Cocktail in Six Countries. J. Agric. Food Chem. 2019, 67, 8096–8106. [Google Scholar] [CrossRef] [Green Version]
- Manojlović, M.S.; Lončarić, Z.; Cabilovski, R.R.; Popović, B.; Karalić, K.; Ivezić, V.; Ademi, A.; Singh, B.R. Biofortification of wheat cultivars with selenium. Acta Agric. Scand. Sect. B Soil Plant. Sci. 2019, 69, 715–724. [Google Scholar] [CrossRef]
- Ramkissoon, C.; Degryse, F.; da Silva, R.C.; Baird, R.; Young, S.D.; Bailey, E.H.; McLaughlin, M.J. Improving the efficacy of selenium fertilizers for wheat biofortification. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- de Lima Lessa, J.H.; Araujo, A.M.; Ferreira, L.A.; da Silva Júnior, E.C.; de Oliveira, C.; Corguinha, A.P.B.; Martins, F.A.D.; de Carvalho, H.W.P.; Guilherme, L.R.G.; Lopes, G. Agronomic biofortification of rice (Oryza sativa L.) with selenium and its effect on element distributions in biofortified grains. Plant. Soil 2019, 444, 331–342. [Google Scholar] [CrossRef]
- Huang, G.; Ding, C.; Yu, X.; Yang, Z.; Zhang, T.; Wang, X. Characteristics of Time-Dependent Selenium Biofortification of Rice (Oryza sativa L.). J. Agric. Food Chem. 2018, 66, 12490–12497. [Google Scholar] [CrossRef]
- Longchamp, M.; Castrec-Rouelle, M.; Biron, P.; Bariac, T. Variations in the accumulation, localization and rate of metabolization of selenium in mature Zea mays plants supplied with selenite or selenate. Food Chem. 2015, 182, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, H.P.G.; de Queiroz Barcelos, J.P.; Junior, E.F.; Santos, E.F.; Silva, V.M.; Moraes, M.F.; Putti, F.F.; dos Reis, A.R. Agronomic biofortification of upland rice with selenium and nitrogen and its relation to grain quality. J. Cereal Sci. 2018, 79, 508–515. [Google Scholar] [CrossRef]
- Wang, Y.D.; Wang, X.; Wong, Y.S. Generation of selenium-enriched rice with enhanced grain yield, selenium content and bioavailability through fertilisation with selenite. Food Chem. 2013, 141, 2385–2393. [Google Scholar] [CrossRef]
- Ngigi, P.B.; Lachat, C.; Masinde, P.W.; Du Laing, G. Agronomic biofortification of maize and beans in Kenya through selenium fertilization. Environ. Geochem. Health 2019, 41, 2577–2591. [Google Scholar] [CrossRef]
- Winter, K.A.; Sanderson, J.B.; Gupta, U.C. Selenium content of barley as influenced by selenite- and selenate-enriched fertilizers. Commun. Soil Sci. Plant. Anal. 1993, 24, 1165–1170. [Google Scholar] [CrossRef]
- Rodrigo, S.; Santamaria, O.; Chen, Y.; McGrath, S.P.; Poblaciones, M.J. Selenium speciation in malt, wort, and beer made from selenium-biofortified two-rowed barley grain. J. Agric. Food Chem. 2014, 62, 5948–5953. [Google Scholar] [CrossRef] [PubMed]
- Poblaciones, M.J.; Rodrigo, S.; Santamaría, O.; Chen, Y.; McGrath, S.P. Agronomic selenium biofortification in Triticum durum under Mediterranean conditions: From grain to cooked pasta. Food Chem. 2014, 146, 378–384. [Google Scholar] [CrossRef]
- Ducsay, L.; Ložek, O.; Marček, M.; Varényiová, M.; Hozlár, P.; Lošák, T. Possibility of selenium biofortification of winter wheat grain. Plant. Soil Environ. 2016, 62, 379–383. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Ali, F.; Wang, M.; Dinh, Q.T.; Zhou, F.; Bañuelos, G.S.; Liang, D. Understanding boosting selenium accumulation in Wheat (Triticum aestivum L.) following foliar selenium application at different stages, forms, and doses. Environ. Sci. Pollut. Res. 2020, 27, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liu, K.; Li, M.; Zhang, W.; Zhao, X.; Zhao, Z. Field Crops Research Di ff erence of selenium uptake and distribution in the plant and selenium form in the grains of rice with foliar spray of selenite or selenate at di ff erent stages. Field Crop. Res. 2017, 211, 165–171. [Google Scholar] [CrossRef]
- Pezzarossa, B.; Remorini, D.; Gentile, M.L.; Massai, R. Effects of foliar and fruit addition of sodium selenate on selenium accumulation and fruit quality. J. Sci. Food Agric. 2012, 92, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Kápolna, E.; Hillestrøm, P.R.; Laursen, K.H.; Husted, S.; Larsen, E.H. Effect of foliar application of selenium on its uptake and speciation in carrot. Food Chem. 2009, 115, 1357–1363. [Google Scholar] [CrossRef]
- Winkel, L.H.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef] [Green Version]
- Márquez, V.G.; Moreno, Á.M.; Mendoza, A.B.; Macías, J.M. Ionic selenium and nanoselenium as biofortifiers and stimulators of plant metabolism. Agronomy 2020, 10, 1399. [Google Scholar] [CrossRef]
- Lyons, G. Biofortification of cereals with foliar selenium and iodine could reduce hypothyroidism. Front. Plant Sci. 2018, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Izydorczyk, G.; Ligas, B.; Mikula, K.; Witek-Krowiak, A.; Moustakas, K.; Chojnacka, K. Biofortification of edible plants with selenium and iodine—A systematic literature review. Sci. Total Environ. 2020, 754, 141983. [Google Scholar] [CrossRef]
- Cartes, P.; Gianfreda, L.; Mora, M.L. Uptake of selenium and its antioxidant activity in ryegrass when applied as selenate and selenite forms. Plant. Soil 2005, 276, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Nancharaiah, Y.V.; Lens, P.N.L. Ecology and Biotechnology of Selenium-Respiring Bacteria. Microbiol. Mol. Biol. Rev. 2015, 79, 61–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasin, M.; El Mehdawi, A.F.; Jahn, C.E.; Anwar, A.; Turner, M.F.S.; Faisal, M.; Pilon-Smits, E.A.H. Seleniferous soils as a source for production of selenium-enriched foods and potential of bacteria to enhance plant selenium uptake. Plant. Soil 2014, 386, 385–394. [Google Scholar] [CrossRef]
- White, P.J. Selenium in soils and crops. Mol. Integr. Toxicol. 2018, 29–50. [Google Scholar] [CrossRef]
- Natasha; Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef]
- Mayland, H.F.; James, L.F.; Panter, K.E.; Sonderegger, J.L. Selenium in Seleniferous Environments. Selenium Agric. Environ. 2015, 15–50. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Ren, J.; Xue, C.; Lin, E. Study on the relationship between soil selenium and plant selenium uptake. Plant. Soil 2005, 277, 197–206. [Google Scholar] [CrossRef]
- Hart, D.J.; Fairweather-Tait, S.J.; Broadley, M.R.; Dickinson, S.J.; Foot, I.; Knott, P.; McGrath, S.P.; Mowat, H.; Norman, K.; Scott, P.R.; et al. Selenium concentration and speciation in biofortified flour and bread: Retention of selenium during grain biofortification, processing and production of Se-enriched food. Food Chem. 2011, 126, 1771–1778. [Google Scholar] [CrossRef]
- Li, Z.; Liang, D.; Peng, Q.; Cui, Z.; Huang, J.; Lin, Z. Interaction between selenium and soil organic matter and its impact on soil selenium bioavailability: A review. Geoderma 2017, 295, 69–79. [Google Scholar] [CrossRef]
- Zhang, P.; Sparks, D.L. Kinetics of Selenate and Selenite Adsorption/Desorption at the Goethite/Water Interface. Environ. Sci. Technol. 1990, 24, 1848–1856. [Google Scholar] [CrossRef]
- Ramkissoon, C.; Degryse, F.; Young, S.; Bailey, E.H.; McLaughlin, M.J. Effect of soil properties on time-dependent fixation (ageing) of selenate. Geoderma 2021, 383, 114741. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y.; Li, J.; Wan, Y.; Huang, Q.; Guo, Y.; Li, H. Effects of Different Forms of Selenium Fertilizers on Se Accumulation, Distribution, and Residual Effect in Winter Wheat-Summer Maize Rotation System. J. Agric. Food Chem. 2017, 65, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Girling, C.A. Selenium in agriculture and the environment. Agric. Ecosyst. Environ. 1984, 11, 37–65. [Google Scholar] [CrossRef]
- Mikkelsen, R.L.; Page, A.L.; Bingham, F.T. Factors Affecting Selenium Accumulation by Agricultural Crops. In Selenium in Agriculture and the Environment; Jacobs, L.W., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1989; pp. 65–94. [Google Scholar]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.T.; Li, Z.; Tran, T.A.T.; Wang, D.; Liang, D. Role of organic acids on the bioavailability of selenium in soil: A review. Chemosphere 2017, 184, 618–635. [Google Scholar] [CrossRef] [PubMed]
- Eich-Greatorex, S.; Sogn, T.A.; Øgaard, A.F.; Aasen, I. Plant availability of inorganic and organic selenium fertiliser as influenced by soil organic matter content and pH. Nutr. Cycl. Agroecosyst. 2007, 79, 221–231. [Google Scholar] [CrossRef]
- Zieve, R.; Peterson, P.J. Factors influencing the volatilization of selenium from soil. Sci. Total Environ. 1981, 19, 277–284. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium Biofortification and Interaction With Other Elements in Plants: A Review. Front. Plant Sci. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Xing, G.; Tang, S.; Pang, Y.; Yi, Q.; Huang, Q.; Huang, X.; Huang, J.; Li, P.; Fu, H. Improving soil selenium availability as a strategy to promote selenium uptake by high-Se rice cultivar. Environ. Exp. Bot. 2019, 163, 45–54. [Google Scholar] [CrossRef]
- Malagoli, M.; Schiavon, M.; Dall’Acqua, S.; Pilon-Smits, E.A.H. Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 2015, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, N.; Akhtar, M.; Kamran, M.A.; Imran, M.; Riaz, M.A.; Kamran, K.; Hussain, S. Selenium biofortification in food crops: Key mechanisms and future perspectives. J. Food Compos. Anal. 2020, 93, 103615. [Google Scholar] [CrossRef]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Wiesner-Reinhold, M.; Schreiner, M.; Baldermann, S.; Schwarz, D.; Hanschen, F.S.; Kipp, A.P.; Rowan, D.D.; Bentley-Hewitt, K.L.; McKenzie, M.J. Mechanisms of selenium enrichment and measurement in brassicaceous vegetables, and their application to human health. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, M.; Pilon-Smits, E.A.H. The fascinating facets of plant selenium accumulation—Biochemistry, physiology, evolution and ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef] [Green Version]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium Biofortification of Agricultural Crops and Effects on Plant Nutrients and Bioactive Compounds Important for Human Health and Disease Prevention—A Review. Plant. Foods Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Läuchli, A. Selenium in Plants: Uptake, Functions, and Environmental Toxicity. Bot. Acta 1993, 106, 455–468. [Google Scholar] [CrossRef]
- Funes-Collado, V.; Morell-Garcia, A.; Rubio, R.; López-Sánchez, J.F. Selenium uptake by edible plants from enriched peat. Sci. Hortic. 2013, 164, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol. Plant. 2015, 37. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, M.; Lima, L.W.; Jiang, Y.; Hawkesford, M.J. Effects of Selenium on Plant Metabolism and Implications for Crops and Consumers. In Selenium in Plants; Springer: Cham, Switzerland, 2017; pp. 257–275. [Google Scholar] [CrossRef]
- Pedrero, Z.; Madrid, Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: A review. Anal. Chim. Acta 2009, 634, 135–152. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, P.J. Selenium metabolism in plants. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.H.; Quinn, C.F. Selenium metabolism in plants. In Cell Biology of Metals and Nutrients; Springer: Berlin/Heidelberg, Germany, 2010; pp. 225–241. [Google Scholar]
- Pilon-Smits, E.A.H. Selenium in Plants. In Scientific American; Springer International Publishing: Cham, Switzerland, 2015; Volume 8, p. 2957. ISBN 9783319088075. [Google Scholar]
- El-Ramady, H.; Abdalla, N.; Alshaal, T.; El-Henawy, A.; Faizy, S.E.D.A.; Shams, M.S.; Shalaby, T.; Bayoumi, Y.; Elhawat, N.; Shehata, S.; et al. Selenium and its role in higher plants. In Pollutants in Buildings, Water and Living Organisms; Springer International Publishing: Cham, Switzerland, 2015; pp. 235–296. ISBN 9783319192765. [Google Scholar]
- Jha, A.B.; Warkentin, T.D. Biofortification of pulse crops: Status and future perspectives. Plants 2020, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Qu, J.; Pu, Y.; Rao, S.; Xu, F.; Wu, C. Selenium biofortification of crop food by beneficial microorganisms. J. Fungi 2020, 6, 59. [Google Scholar] [CrossRef]
- Patel, P.J.; Trivedi, G.R.; Shah, R.K.; Saraf, M. Selenorhizobacteria: As biofortification tool in sustainable agriculture. Biocatal. Agric. Biotechnol. 2018, 14, 198–203. [Google Scholar] [CrossRef]
- Yang, X.E.; Chen, W.R.; Feng, Y. Improving human micronutrient nutrition through biofortification in the soil-plant system: China as a case study. Environ. Geochem. Health 2007, 29, 413–428. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Shamberger, R.J. Selenium in the environment. Sci. Total Environ. 1981, 17, 59–74. [Google Scholar] [CrossRef]
- Lyons, G.H.; Stangoulis, J.C.R.; Graham, R.D. Exploiting micronutrient interaction to optimize biofortification programs: The case for inclusion of selenium and iodine in the HarvestPlus program. Nutr. Rev. 2004, 62, 247–252. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Zhao, F.J. Strategies for increasing the selenium content of wheat. J. Cereal Sci. 2007, 46, 282–292. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błazejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, G.; Stangoulis, J.; Graham, R. High-selenium wheat: Biofortification for better health. Nutr. Res. Rev. 2003, 16, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Schiavon, M.; Nardi, S.; dalla Vecchia, F.; Ertani, A. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant. Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Rawat, N.; Tiwari, V.K.; Singh, N.; Randhawa, G.S.; Singh, K.; Chhuneja, P.; Dhaliwal, H.S. Evaluation and utilization of Aegilops and wild Triticum species for enhancing iron and zinc content in wheat. Genet. Resour. Crop. Evol. 2009, 56, 53–64. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop wild relatives (Cwr) priority in italy: Distribution, ecology, in situ and ex situ conservation and expected actions. Sustainability 2021, 13, 1682. [Google Scholar] [CrossRef]
- Brown, K.; Arthur, J. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Pilon-Smits, E.A.H.; Zhao, F.J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant. Sci. 2009, 14, 436–442. [Google Scholar] [CrossRef]
- Osendarp, S.J.M.; Martinez, H.; Garrett, G.S.; Neufeld, L.M.; De-Regil, L.M.; Vossenaar, M.; Darnton-Hill, I. Large-Scale Food Fortification and Biofortification in Low- and Middle-Income Countries: A Review of Programs, Trends, Challenges, and Evidence Gaps. Food Nutr. Bull. 2018, 39, 315–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephen, R.C.; Saville, D.J.; Watkinson, J.H. The effects of sodium selenate applications on growth and selenium concentration in wheat. N. Z. J. Crop. Hortic. Sci. 1989, 17, 229–237. [Google Scholar] [CrossRef]
- Mao, H.; Wang, J.; Wang, Z.; Zan, Y.; Lyons, G.; Zou, C. Using agronomic biofortification to boost zinc, selenium, and iodine concentrations of food crops grown on the loess plateau in China. J. Soil Sci. Plant. Nutr. 2014, 14, 459–470. [Google Scholar] [CrossRef] [Green Version]
- El-Ramady, H.; Abdalla, N.; Taha, H.S.; Alshaal, T.; El-Henawy, A.; Faizy, S.E.D.A.; Shams, M.S.; Youssef, S.M.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016, 14, 123–147. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, K.S. Role of nano-selenium in health and environment. J. Biotechnol. 2020, 325, 152–163. [Google Scholar] [CrossRef]
- Lara, T.S.; de Lima Lessa, J.H.; de Souza, K.R.D.; Corguinha, A.P.B.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium biofortification of wheat grain via foliar application and its effect on plant metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- Petković, K.P.; Anojlović, M.M.; Abilovski, R.Č.; Rstić, Đ.K. Foliar Application Of Selenium, Zinc And Copper In Alfalfa (Medicago sativa L.) Biofortification. Turk. J. Filed Crop. 2019, 24, 81–90. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Mao, H.; Zhao, H.; Huang, D. Increasing Se concentration in maize grain with soil- or foliar-applied selenite on the Loess Plateau in China. Field Crop. Res. 2013, 150, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Lidon, F.C.; Oliveira, K.; Ribeiro, M.M.; Pelica, J.; Pataco, I.; Ramalho, J.C.; Leitão, A.E.; Almeida, A.S.; Campos, P.S.; Ribeiro-Barros, A.I.; et al. Selenium biofortification of rice grains and implications on macronutrients quality. J. Cereal Sci. 2018, 81, 22–29. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Chilimba, A.D.C.; Young, S.D.; Black, C.R.; Meacham, M.C.; Lammel, J.; Broadley, M.R. Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crop. Res. 2012, 125, 118–128. [Google Scholar] [CrossRef]
- Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Tahir, M.N.; Zulfiqar, B.; Salahuddin, M.; Shabbir, R.N.; Aslam, M. Selenium supplementation affects physiological and biochemical processes to improve fodder yield and quality of maize (Zea mays L.) under water deficit conditions. Front. Plant Sci. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, C.; Park, Y.H.; Myoung, K.H.; Suh, M.K.; McArthur, T.; Lyons, G.; Stewart, D. The Bio-fortification of Barley with Selenium Catherine. In Proceedings of the Institute of Brewery & Distillating (Asia-Pacific Section) Carventron, Hobart, Tasmania, 19–24 March 2006. [Google Scholar]
- Wang, S.; Liang, D.; Wang, D.; Wei, W.; Fu, D.; Lin, Z. Selenium fractionation and speciation in agriculture soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Sci. Total Environ. 2012, 427–428, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–51. [Google Scholar] [CrossRef]
- Shanker, K.; Mishra, S.; Srivastava, S.; Srivastava, R.; Daas, S.; Prakash, S.; Srivastava, M.M. Effect of selenite and selenate on plant uptake and translocation of mercury by tomato (Lycopersicum esculentum). Plant. Soil 1996, 183, 233–238. [Google Scholar] [CrossRef]
- Guerrero, B.; Llugany, M.; Palacios, O.; Valiente, M. Dual effects of different selenium species on wheat. Plant. Physiol. Biochem. 2014, 83, 300–307. [Google Scholar] [CrossRef]
- Cubadda, F.; Aureli, F.; Ciardullo, S.; D’Amato, M.; Raggi, A.; Acharya, R.; Reddy, R.A.V.; Prakash, N.T. Changes in selenium speciation associated with increasing tissue concentrations of selenium in wheat grain. J. Agric. Food Chem. 2010, 58, 2295–2301. [Google Scholar] [CrossRef]
- Yin, H.; Qi, Z.; Li, M.; Ahammed, G.J.; Chu, X.; Zhou, J. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol. Environ. Saf. 2019, 169, 911–917. [Google Scholar] [CrossRef]
- Lidon, F.C.; Oliveira, K.; Galhano, C.; Guerra, M.; Ribeiro, M.M.; Pelica, J.; Pataco, I.; Ramalho, J.C.; Leitaõ, A.E.; Almeida, A.N.A.S.; et al. Selenium biofortification of rice through foliar application with selenite and selenate. Exp. Agric. 2019, 55, 528–542. [Google Scholar] [CrossRef]
- Perrino, E.V.; Calabrese, G. Endangered segetal species in southern Italy: Distribution, conservation status, trends, actions and ethnobotanical notes. Genet. Resour. Crop. Evol. 2018, 65, 2107–2134. [Google Scholar] [CrossRef]
- Hammer, K.; Gladis, T.; Diederichsen, A. Weeds as genetic resources. Plant. Genet. Rersour. Newsl. 1997, 111, 33–39. [Google Scholar]
| Specie | Type of Experiment | Application | Time of Application | Se Form | g Se/ha | Control µg·kg−1 | Se Content in Grain µg·kg−1 DW | Increase | Increase by 1 g of Added Se | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Rice | Field experiment | Soil | At heading | Selenite | 30 | 76 | 59 | 0.78 | 0.03 | [19] |
| Rice | Field experiment | Soil | At heading | Selenate | 30 | 76 | 79 | 1.04 | 0.03 | [19] |
| Rice | Field experiment | Soil | At heading | Selenite | 30 | 86 | 85 | 0.99 | 0.03 | [19] |
| Rice | Field experiment | Soil | At heading | Selenate | 30 | 86 | 92 | 1.07 | 0.04 | [19] |
| Rice | Field experiment | Soil | At heading | Selenite | 30 | 97 | 82 | 0.85 | 0.03 | [19] |
| Rice | Field experiment | Soil | At heading | Selenate | 30 | 97 | 92 | 0.95 | 0.03 | [19] |
| Rice | Field experiment | Foliar | At heading | Selenite | 30 | 76 | 273 | 3.59 | 0.12 | [19] |
| Rice | Field experiment | Foliar | At heading | Selenate | 30 | 76 | 150 | 1.97 | 0.07 | [19] |
| Rice | Field experiment | Foliar | At heading | Selenite | 30 | 86 | 122 | 1.42 | 0.05 | [19] |
| Rice | Field experiment | Foliar | At heading | Selenate | 30 | 86 | 105 | 1.22 | 0.04 | [19] |
| Rice | Field experiment | Foliar | At heading | Selenite | 30 | 97 | 136 | 1.4 | 0.05 | [19] |
| Rice | Field experiment | Foliar | At heading | Selenate | 30 | 97 | 176 | 1.81 | 0.06 | [19] |
| Rice | Field experiment, no till | Soil | At sowing | Selenate | 25 | 30 | 320 | 10.67 | 0.43 | [28] |
| Rice | Plastic pots in growth chamber | Foliar | Seven times through vegetation | Selenite | 0.53 | 30 | 100 | 3.33 | 6.35 | [29] |
| Rice | Plastic pots in growth chamber | Foliar | Seven times through vegetation | Selenite | 10.5 | 30 | 1540 | 51.33 | 4.89 | [29] |
| Rice | Plastic pots in growth chamber | Foliar | Seven times through vegetation | Selenite | 21 | 30 | 1560 | 52 | 2.48 | [29] |
| Maize | Field experiment | Soil | Before sowing | Selenite | 150 | 3.7 | 51 | 13.78 | 0.09 | [106] |
| Maize | Field experiment | Foliar | Tasseling and one week after silking | Selenite | 11 | 11 | 96 | 8.73 | 0.79 | [106] |
| Maize | Field experiment | Soil | Before seeding | Selenate | 5 | 34 | 41.66 | 1.23 | 0.25 | [30] |
| Maize | Field experiment | Soil | Before seeding | Selenate | 10 | 34 | 68.33 | 2.01 | 0.2 | [30] |
| Maize | Field experiment | Soil | Before seeding | Selenate | 20 | 34 | 92.66 | 2.73 | 0.14 | [30] |
| Maize | Field experiment | Foliar | During the stem elongation stage | Selenate | 5 | 34 | 156.66 | 4.61 | 0.92 | [30] |
| Maize | Field experiment | Foliar | During the stem elongation stage | Selenate | 10 | 34 | 205.33 | 6.04 | 0.6 | [30] |
| Maize | Field experiment | Foliar | During the stem elongation stage | Selenate | 20 | 34 | 305.66 | 8.99 | 0.45 | [30] |
| Barley | Field experiment | Soil | Before seeding | Selenite | 20 | 45 | 57 | 1.27 | 0.06 | [31] |
| Barley | Field experiment | Soil | Before seeding | Selenate | 20 | 33 | 391 | 11.85 | 0.59 | [31] |
| Barley | Field experiment | Soil | Before seeding | Selenite | 40 | 45 | 76 | 1.69 | 0.04 | [31] |
| Barley | Field experiment | Soil | Before seeding | Selenate | 40 | 33 | 959 | 29.06 | 0.73 | [31] |
| Barley | Field experiment | Foliar | End of tillering EC-39 | Selenate | 10 | 111.7 | 880 | 7.88 | 0.79 | [32] |
| Barley | Field experiment | Foliar | End of tillering EC-39 | Selenate | 20 | 111.7 | 1113.9 | 9.97 | 0.5 | [32] |
| Barley | Field experiment | Foliar | End of tillering EC-39 | Selenite | 10 | 111.7 | 270 | 2.42 | 0.24 | [32] |
| Barley | Field experiment | Foliar | End of tillering EC-39 | Selenite | 20 | 111.7 | 345.5 | 3.09 | 0.15 | [32] |
| Wheat | Field experiment | Foliar | Tillering state | Selenite | 10 | 66.6 | 153.6 | 2.31 | 0.23 | [33] |
| Wheat | Field experiment | Foliar | Tillering state | Selenite | 20 | 66.6 | 254.8 | 3.83 | 0.19 | [33] |
| Wheat | Field experiment | Foliar | Tillering state | Selenite | 40 | 66.6 | 430.4 | 6.46 | 0.16 | [33] |
| Wheat | Field experiment | Foliar | Tillering state | Selenate | 10 | 66.6 | 266.8 | 4.01 | 0.4 | [33] |
| Wheat | Field experiment | Foliar | Tillering state | Selenate | 20 | 66.6 | 820 | 12.31 | 0.62 | [33] |
| Wheat | Field experiment | Foliar | Tillering state | Selenate | 40 | 66.6 | 1383.2 | 20.77 | 0.52 | [33] |
| Wheat | Small-plot field experiment | Foliar | Growth stage of 2nd node on the main stem | Selenite | 10 | 32 | 51 | 1.59 | 0.16 | [34] |
| Wheat | Small-plot field experiment | Foliar | Growth stage of 2nd node on the main stem | Selenite | 20 | 32 | 72 | 2.25 | 0.11 | [34] |
| Wheat | Small-plot field experiment | Foliar | Growth stage of 2nd node on the main stem | Selenate | 10 | 32 | 190 | 5.94 | 0.59 | [34] |
| Wheat | Small-plot field experiment | Foliar | Growth stage of 2nd node on the main stem | Selenate | 20 | 32 | 350 | 10.94 | 0.55 | [34] |
| Wheat | Field experiment | Foliar | Preflowering | Selenite | 20 | 120 | 610 | 5.08 | 0.25 | [35] |
| Wheat | Field experiment | Foliar | Preflowering | Selenate | 20 | 120 | 1340 | 11.17 | 0.56 | [35] |
| Wheat | Field experiment | Foliar | Pre-grain filling stages | Selenite | 20 | 120 | 970 | 8.08 | 0.4 | [35] |
| Wheat | Field experiment | Foliar | Pre-grain filling stages | Selenate | 20 | 120 | 1590 | 13.25 | 0.66 | [35] |
| Wheat | Field experiment | Foliar | During GS 31 and GS 49 stage | Selenate | 5 | 150 | 640 | 4.27 | 0.85 | [20] |
| Wheat | Field experiment | Foliar | During GS 31 and GS 49 stage | Selenate | 25 | 150 | 2390 | 15.93 | 0.64 | [20] |
| Wheat | Field experiment | Foliar | During GS 31 and GS 49 stage | Selenate | 50 | 150 | 2820 | 18.8 | 0.38 | [20] |
| Wheat | Field experiment | Foliar | During GS 31 and GS 49 stage | Selenate | 80 | 150 | 3930 | 26.2 | 0.33 | [20] |
| Factor | Application Type × Se Form | Species × Se Form | Type of Experiment | Residual |
|---|---|---|---|---|
| Variance | 0.012352 | 0.006132 | 10.624367 | 0.213215 |
| Coefficients of random effects | ||||
| Soil/selenate | −0.007177254 | – | – | |
| Foliar/selenate | 0.095549031 | – | – | |
| Soil/selenite | −0.060080039 | – | – | n.a. |
| Foliar/selenite | −0.022608397 | – | – | |
| Barley/selenate | – | 0.031985788 | – | |
| Maize/selenate | – | 0.012493402 | – | |
| Rice/selenate | – | −0.039315129 | – | |
| Wheat/selenate | – | 0.038706521 | – | |
| Barley/selenite | – | −0.013442636 | – | |
| Maize/selenite | – | 0.010163323 | – | |
| Rice/selenite | – | −0.028004366 | – | |
| Wheat/selenite | – | −0.009765509 | – | |
| Field experiment | – | – | 0.2971243 | |
| Plastic pots in growth chamber | – | – | 4.5913276 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galić, L.; Vinković, T.; Ravnjak, B.; Lončarić, Z. Agronomic Biofortification of Significant Cereal Crops with Selenium—A Review. Agronomy 2021, 11, 1015. https://doi.org/10.3390/agronomy11051015
Galić L, Vinković T, Ravnjak B, Lončarić Z. Agronomic Biofortification of Significant Cereal Crops with Selenium—A Review. Agronomy. 2021; 11(5):1015. https://doi.org/10.3390/agronomy11051015
Chicago/Turabian StyleGalić, Lucija, Tomislav Vinković, Boris Ravnjak, and Zdenko Lončarić. 2021. "Agronomic Biofortification of Significant Cereal Crops with Selenium—A Review" Agronomy 11, no. 5: 1015. https://doi.org/10.3390/agronomy11051015
APA StyleGalić, L., Vinković, T., Ravnjak, B., & Lončarić, Z. (2021). Agronomic Biofortification of Significant Cereal Crops with Selenium—A Review. Agronomy, 11(5), 1015. https://doi.org/10.3390/agronomy11051015