1. Introduction
Veterinary drugs such as anthelmintics represent neglected but risky environmental contaminants. The anthelmintic drugs albendazole (ABZ), ivermectin (IVM), and monepantel (MOP) are regularly administered to livestock to control infections caused by nematodes. The residues of the parent drug, as well as their metabolites, are excreted in faeces or urine and released into the environment. Fields are contaminated by manure, and grasslands or meadows are contaminated directly by excrements of the grazing livestock.
ABZ is metabolized in sheep mostly to ABZ-sulphoxide and ABZ-sulphone [
1,
2]. The MOP is metabolized to MOP-sulphone, presenting the major metabolite, and 13 other metabolites of MOP were detected in faeces or urine of sheep [
2]. Although 16 metabolites of IVM were formed in ovine hepatocytes, the extent of IVM biotransformation is very low as the IVM metabolites present only a small portion when compared to the parent compound [
3]. In our previous in vivo study, operating with recommended IVM doses, we found that IVM is released to the environment in very low concentrations. Eight times lower concentration of IVM (C
max = 0.93 µg·g
−1) was detected in ovine excrements [
4] compared to ABZ (C
max = 7.7 µg·g
−1) [
1].
The residual anthelmintics and their metabolites released to the environment are further absorbed and accumulated by plants and may have a harmful impact. It was also suggested that plants might play an important role in the detoxification of arable land contaminated by these pharmaceutics [
5]. After the xenobiotics enter the plants, plants employ a sophisticated detoxification system that comprises biotransformation and transport of xenobiotics [
6]. This plant defense system includes several xenobiotic-metabolizing enzymes and the enzymes reducing oxidative stress, such as peroxidases, superoxide dismutase, catalase, and glutathione-cooperating enzymes [
7,
8,
9,
10]. Xenobiotic-mediated oxidative stress may be manifested by various changes, including increased lipid peroxidation [
11] and/or proline accumulation [
12].
Previous reports [
13,
14,
15,
16] prove the accumulation and biotransformation of anthelmintics in the roots as well as in the shoots in higher plants. Our previous study detected ABZ-sulfoxide, ABZ-sulfone, converted via S-oxidation, and glucosides formed via the conjugation of ABZ and ABZ-sulfoxide with UDP-glucose, as the main metabolites of ABZ in
Campanula rotundifolia [
13] and
Plantago lanceolata [
14]. Regarding the IVM, its biotransformation consists of demethylation and hydroxylation, i.e., only phase I detoxification reactions [
6,
9] in
P. lanceolata with the hydroxylated IVM being the major metabolite [
15]. The study in
Medicago sativa and
P. lanceolata plants revealed that most of the MOP metabolites were formed via two-step S-oxidation, reduction of carbonyl group, nitrile hydrolyses, and their combinations. Further, these metabolites were conjugated with glucose or sulfate [
16]. All the above-mentioned studies were performed using the in vitro cultivated plant models exposed to the solutions of parent compounds in higher concentrations in order to detect all metabolites. However, in real conditions, plants meet anthelmintics in much lower concentration and in a mixture of residual parent compounds and their metabolites generated by livestock.
To get closer to real conditions, the present study was designed to test the uptake, accumulation, metabolism, and effects of anthelmintics and their metabolites present in manure from treated sheep using clover
(Trifolium pratense), a common fodder plant. We decided to test if the manure from anthelmintic-treated livestock is safe for higher plants with no harmful impact via the increased generation of reactive oxygen species. Moreover, we would like to know what quantity of parent compounds and their metabolites are accumulated from excrements into the plant shoots, since it might have a further negative impact on increasing resistance of helminths infecting the gastrointestinal tract of grazing livestock [
17]. Our present study includes three widely available broad-spectrum anthelmintics representing three classes with different structure, mode of action, as well as pharmacokinetic behavior: benzimidazole ABZ, macrocyclic lactone IVM, and amino-acetonitrile derivative MOP. We used clover plants grown in the greenhouse and fertilized with excrements from sheep treated with ABZ, IVM, or MOP. The uptake and biotransformation of anthelmintics and their metabolites by plants were analyzed using UHPLC-MS/MS. To evaluate the potential phytotoxicity of anthelmintics, we monitored parameters, such as changes in proline accumulation, lipid peroxidation, hydrogen peroxide accumulation, and changes in the activity of antioxidative enzymes involved in the plant defense system.
2. Materials and Methods
2.1. Chemicals and Reagents
The ABZ suspension Aldifal was obtained from Mikrochem s.r.o. (Pezinok, Slovakia). The IVM form of an injection, Noromectin (Norbrook Laboratories Ltd., Monaghan, Ireland) was used. MOP solution Zolvix ™ was obtained from Elanco Buenos Aires, Argentina. Nitrazon (Rhizobium mix) was purchased from Farma Žiro, s.r.o. (Nehvizdy, Czech Republic). Chemical standards of mebendazole (MBZ), ABZ, ABZ sulfoxide (ABZ-SO), ABZ sulfone (ABZ-SO2), IVM, doramectin (DOR), and L-proline were supplied by Sigma-Aldrich (Prague, Czech Republic) in analytical standard quality ≥98%. MOP and MOP sulfone (MOP-SO2) were purchased by Toronto Research Chemicals Inc (Toronto, ON, Canada) in analytical standard quality ≥98%. Acetonitrile (ACN) (UHPLC-MS quality) and ethyl acetate (HPLC quality) were purchased from VWR International s.r.o. (Prague, Czech Republic). Bradford reagent and protein standard bovine serum albumin (BSA) were purchased from Bio-Rad s.r.o. (Prague, Czech Republic). Other chemicals, solvents, and reagents, such as hydrogen peroxide, trichloroacetic acid, thiobarbituric acid, malondialdehyde, ethanol, ninhydrin, acetic acid, ethylenediaminetetraacetic acid, polyvinylpyrrolidone K 30, and Triton™ X-100, were obtained from Sigma-Aldrich s.r.o. (Prague, Czech Republic).
2.2. Animals, Treatment with Anthelmintics
The faeces were collected from sheep (Texel breed, males, 7–9 months old; farm Dibaq a.s., Helvíkovice, Chzech Republic) 24–48 h after the treatment with anthelmintic drugs in the recommended dosages per body weight (b.w.) and mixed in 1:1 proportion (from two collecting periods). One group of sheep was treated with suspension Aldifal (ABZ, 10 mg·kg
−1 b.w.), the second group with Zolvix ™ (MOP 2.5 mg·kg
−1 b.w.), and the third group was treated subcutaneously with Noromectin (IVM 0.2 mg·kg
−1 b.w.). Faeces samples containing ivermectin were used to determine pharmacokinetics in the faeces of sheep in a previous work described by Vokral et al. [
4] and were used for this study. Faeces from untreated lambs/sheep were used as a control. All faeces collected from sheep were stored at −20 °C until applied to plants.
2.3. Preparation of Standards, Working Solutions, Calibration Standards
For calibration curve preparation, the anthelmintics and metabolites were dissolved in the mobile phase as well as in the matrix to avoid matrix effects. For ABZ related compounds, the calibration curves were prepared at eight calibration points with concentrations ranging from 0.002653 to 1.326 µg·mL
−1 for ABZ, 0.002813–1.4065 µg·mL
−1 for ABZ-SO, and 0.002973–1.4865 µg·mL
−1 for ABZ-SO
2. For MOP related compounds, the calibration curves were prepared at eight calibration points with concentrations ranging from 0.237 to 1.893 µg·mL
−1 for MOP and 0.25269–2.021 µg·mL
−1 for MOP-SO
2. MBZ (internal standard) was present in each calibration sample in the concentration 1.476 µg·mL
−1. The sample preparation procedure used for calibration samples was the same as in
Section 2.6. Relevant information regarding IVM is available in the article written by Vokral et al. [
4].
2.4. The Extraction of Anthelmintics from Feaces for LC/MS Analysis
Prior to the extraction, the faeces samples needed to be dried in the oven at 30 °C to unify the moisture content. For ABZ, the extraction method was based on the two-step LLE extraction technique. One gram of dry samples of faeces was transferred into 50 mL tubes with 13 mL of water (pH = 10). Then, the internal standard was added (10 µL, 100 µM). The samples were shaken (30 min) in multi-vortexer Multi Reax (Heidolph, Schwabach, Germany). Afterwards, ethyl acetate (27 mL) was added and then shaken (1 h), followed by centrifugation (3000× g; 20 min) in Eppendorf centrifuge 5810 R. The upper organic layer (21 mL) was gradually evaporated to dryness in 5 mL centrifugation tubes in Eppendorf Concentrator plus (Eppendorf, Hamburg, Germany) at 30 °C. The extraction procedure was repeated and both supernatants (42 mL) were pooled in one tube. All samples were stored at −20 °C.
A simple extraction method with acetonitrile was used for the MOP. One gram of dry faeces was transferred into 15 mL tubes with 5 mL of water and IS standard (10 µL, 100 µM) was added. The samples were shaken (30 min) in a multi-vortexer Multi Reax (Heidolph, Schwabach, Germany). Then, acetonitrile (6 mL) was added, again vigorously shaken (30 min), followed by sonication in the water bath (10 min) and with centrifugation (3000× g; 20 min) in Eppendorf centrifuge 5810 R. The upper layer (4.5 mL) was then transferred into 5 mL centrifugation tubes and evaporated to dryness in a vacuum rotary evaporator at 30 °C. The extraction step was repeated once more with fresh acetonitrile. All samples were stored at −20 °C.
The extraction method for IVM is further described in Vokral et al. [
4].
2.5. Plant Material, Growth Conditions/Stress Treatments, and Extraction
The seeds of Trifolium pratense (tetraploid var. Beskyd), purchased from AROS-osiva s.r.o. (Prague, Czech Republic), were sowed using commercial compost Agro Profi from Agro CS a.s. (Říkov, Czech Republic) into pots (15 × 15 × 20 cm) in a greenhouse (air-conditioned to 23 °C, relative humidity 60%). The day light was supplemented with artificial light (sodium discharge lamps, 400 W, an average irradiation of 72 µmol∙m−2 at the plants’ surfaces, and the horizontal differences in irradiation were <20%) to maintain the photoperiod 16/8 h. One week after sowing, the soil was supplemented with nitrazon (Rhizobium mix; Farma Žiro, s.r.o., Nehvizdy, Czech Republic). After three weeks, 9 g of faeces (of treated or untreated sheep) were added into each pot. The faeces were evenly dispersed on the soil surface. The plants cultivated in soil with faeces of untreated sheep were used as a control. Plants were regularly watered with tap water on the top of the substrate in order to allow continuous decomposition of faeces to the substrate and to keep an optimal moisture of 40–60%.
Plant samples (clover shoots) were collected 3, 10, 21, and 42 days after applying the faeces. Shoots were placed immediately into liquid nitrogen and stored under −80 °C until analyses for stress markers. Two independent experiments (cultivations) were performed.
The plant tissue samples (35–50 mg) were homogenized (2 × 40 s; 6 m/s) with ultrapure-water (2.5 mL; pH adjusted with ammonia to 10) using the beads homogenizer FastPrep-24 (MP Biomedicals, Santa Ana, CA, USA). Prior to extraction, proper internal standard DOR or MBZ (100 µM, × 10 µL) were added to the samples. The homogenates were subjected to liquid-liquid extraction (LLE) with ethyl acetate (7.5 mL) as an extraction solvent. Then, the samples were shaken (30 min) in the multi-vortexer Multi Reax (Heidolph, Schwabach, Germany) and centrifugated (3000× g; 15 min) in Eppendorf centrifuge 5810 R (Eppendorf, Germany). The ethyl acetate (5 mL) was transferred into an HPLC vial and evaporated to dryness (30 °C) using Eppendorf Concentrator plus (Eppendorf, Hamburg, Germany). All samples were stored at −20 °C.
2.6. UHPLC-MS/MS Analysis–Instrumental and Operating Conditions
Prior to analyses, the dried samples were reconstituted in ACN (30 µL), vortexed (30 s), and ultra-pure water (70 µL) was then added. The mixture was vortexed (30 s) and sonicated (5 min). The samples were filtered through PTFE syringe filters (4 mm; 0.22 µm) and placed into glass vials with inserts. The standard samples underwent the same procedure. A UHPLC Shimadzu Nexera coupled with Shimadzu LCMS-8030 (Shimadzu, Kyoto, Japan) triple quadrupole mass spectrometer was employed to analyse all the samples. A Zorbax RRHD Eclipse Plus C18 150 mm × 2.1 mm chromatographic column (Agilent Technologies, Waldbronn, Germany), packed with 1.8 µm particles and precolumn Zorbax RRHD Eclipse Plus C18 5 mm × 2.1 mm, was used for all separations. Three different chromatography and mass spectrometry conditions were used because of the different physical-chemical properties of the compounds of interest. For IVM samples, the UHPLC-MS conditions are completely described by Vokral et al. [
4]. The chromatographic conditions for ABZ and MOP are described in
Section 2.6.1 and
Section 2.6.2. The chromatographic conditions for IVM are described completely by Vokral et al. [
4].
An internal calibration method was used to determine the amount of the compounds of concern in faeces. This method could not be applied to plant samples because the amount of the compounds was below the quantification limits of the methods. Therefore, a method of semi-quantification based on the response factor was chosen. The response factor is determined as the ratio of the peak area of the sample to the peak area of the internal standard.
Mebendazole (MBZ) was used as an internal standard (IS) for normalization of the peak areas of the compounds to correct errors created in the sample preparation steps. The data were analysed using LabSolution LCMS software ver. 5.93 (Shimadzu, Kyoto, Japan). Biological and chemical blank samples were prepared.
2.6.1. ABZ UHPLC-MS Conditions
The analytical column was kept under the temperature of 40 °C. The flow rate was set at 0.4 mL/min, and injection volume at 1 µL. The composition of the mobile phase was water (solvent A) and acetonitrile (solvent B), both with an addition of 0.1% formic acid. The gradient elution conditions were set as follows: 0 min 15% B, 8 min 40% B, 10 min 95% B, and 10–11 min 95% B. Equilibration of the gradient at 15% B was held from 11 to 14.5 min. All measurements were carried out in the positive ion ESI mode, as this mode provides better sensitivity for the studied compounds. The mass spectrometer was operated in selected reaction monitoring (SRM) mode by monitoring three selected ion transitions. The mass spectrometric parameters were determined through direct injection of standard solutions of ABZ, ABZ-SO, ABZ-SO2, and MBZ into the instrument.
The optimum MS parameters of the ion source were: capillary voltage 4.5 kV, heat block temperature 400 °C, DL line temperature 250 °C. The flow rates of dying gas and nebulizing gas were set at 13 L/min and 2.5 L/min, respectively. Argon was used as a collision gas with a pressure value of 230 kPa.
2.6.2. MOP UHPLC-MS Conditions
The analytical column was kept under the temperature 40 °C. The flow rate was set at 0.3 mL/min, and injection volume at 1 µL. The mobile phase consisted of 0.005 mol/L ammonium acetate buffer (solvent A) and acetonitrile (solvent B). The gradient elution conditions were set as follows: 0 min 50% B, 7 min 95% B, 7–8 min 95% B. Equilibration of the gradient at 50% B was held from 8 to 10 min. All measurements were carried out in the negative ion ESI mode because it provides better sensitivity for analytes. The mass spectrometer was operated in SRM mode by monitoring three selected ion transitions. The mass spectrometric parameters were determined through a direct infusion of standard solutions of MOP, MOP-SO2, and MBZ into the instrument.
The optimum MS parameters of the ion source were: capillary voltage 4.5 kV, heat block temperature 400 °C, DL line temperature 250 °C. The flow rates of dying gas and nebulizing gas were set at 8 L/min and 3 L/min, respectively. Argon was used as a collision gas with a pressure value of 230 kPa.
2.7. Hydrogen Peroxide Content
The production of reactive oxygen species (ROS) in clover shoots was evaluated via hydrogen peroxide (H
2O
2) concentration. The iodometric assay was used to determine H
2O
2 content [
18]. Fresh leaves (1 g) were homogenized and extracted in 10 mL of 0.1% Trichloroacetic acid (TCA). The H
2O
2 standard solutions were prepared in 0.1% TCA. The extracts were centrifuged (1300×
g, 4 °C, 15 min.; Centrifuge Hettich Universal 32R, Tuttlingen, Germany). The supernatant and standard solutions were added to the reaction mixture. Further, the reaction mixture consisting of the supernatant in 1% TCA, 10 mM phosphate buffer, and 1 M KI (1:2:2
v/
v; pH 7) was incubated in dark at room temperature for 20 min. Each sample has a duplicate where 1 M KI was replaced with deionized H
2O as a reference value to subtract the plant tissue background. After incubation, the absorbance was measured at 350 nm in 96 flat bottom clear UV transparent microplates using a microplate reader (Tecan Infinite 200 PRO, Tecan Austria GmbH, Grödig, Austria). The H
2O
2 was quantified according to calibration curve (0.5; 1.0; 2.5; 5.0; 10.0; 25.0; and 50.0 nM).
2.8. Lipid Peroxidation
The changes in lipid peroxidation in response to anthelmintic metabolites was measured by estimating malondialdehyde (MDA), a product of lipid peroxidation [
19]. The fresh leaves were extracted in 0.1% TCA (as described in
Section 2.7). The extracts were centrifuged (1300×
g, 4 °C, 20 min.; Centrifuge Hettich Universal 32R, Tuttlingen, Germany) and the supernatant was added to the reaction mixture that consists of 20% TCA with 0.5% Thiobarbituric acid (TBA) in a ratio 4:1 (
v/
v), then incubated for 30 min at 95 °C on ice. The reference samples were incubated in the reaction mixture without TBA. The samples were cooled down for 5 min, centrifuged (1300×
g, 4 °C, 10 min), and the absorbance at 532, 600, and 440 nm was measured in the supernatant using a microplate reader. The MDA was calculated according to the following quotations [
20]:
2.9. Proline Content
The proline accumulation was determined from fresh leaves homogenized in 40% (
v/
v) ethanol (500 mg FW in 10 mL of 40% EtOH) and incubated overnight [
21]. After centrifugation, the supernatants were incubated with 1% ninhydrin (
w/
v), 60% acetic acid (
v/
v), and 20% ethanol (
v/
v), in a water bath at 95 °C for 20 min. The mixture was cooled directly after incubation and the absorbance was measured at 520 nm by a microplate reader (Tecan Infinite 200 PRO, Tecan Austria GmbH, Grödig, Austria). Proline quantification was based on a calibration curve (0.04, 0.1, 0.2, 0.4, and 1.0 mM) with a standard L-Proline.
2.10. Enzyme Extraction and Antioxidative Enzyme Activity Assays
Plant samples (1 g FW) were homogenized in 10 mL of chilled 50 mM Potassium phosphate buffer (pH 7.0), supplemented with 0.1 mM Ethylenediaminetetraacetic acid, 1% Polyvinylpyrrolidone K 30, and 0.5% Triton X-100, at 4 °C. The extracts were centrifuged, and the supernatant was used for determining the activities of enzymes involved in antioxidant defense machinery, protecting the plants against oxidative stress damages [
22]. The protein content was determined using a Bradford reagent [
23] and BSA as a protein standard (125–1000 µg × mL
−1).
The activity of guaiacol peroxidase (POX) and catalase (CAT) was assayed according to Hasanuzzaman et al. [
24]. Ascorbate peroxidase (APX) was assayed as described by Nakano and Asada [
25]. Glutathione S-transferase (GST), using 1-Chloro-2,4-dinitrobenzene as substrate, was assayed according to Hossain et al. [
26]. Glutathione reductase (GR) was assayed according to Bonilla et al. [
27] and Carlberg and Mannervik [
28]. The activity was calculated in mM min
−1 mg
−1 of protein and expressed in percent comparing to control (plants cultivated with the manure of untreated sheep). Superoxide dismutase (SOD) was determined according to El-Shabrawi et al. [
29], with the activity calculated in U mg
−1 of protein and expressed in percent comparing to control.
2.11. Statistical Analysis
All analyses were performed out of samples from three individual plants in each treatment and in each experiment (the whole experiment was repeated twice). The samples were measured at least in triplicate with 3–5 technical replicates according to a particular assay. Results were expressed as an arithmetic mean ± SD (n = 3) indicated with error bars in charts. Data were analyzed by STATISTICA software (StatSoft, TIBCO Software Inc.), subjected to t-test to determine the statistical difference of each particular treatment individually in comparison to control at p ≤ 0.01 or p ≤ 0.05 according to the data set. For the UHPLC-MS/MS analysis, all samples were prepared in triplicate, with the data presented as an arithmetic mean ± SD (n = 3).
4. Discussion
The increased generation of reactive oxygen species (ROS) is generally a known response of plants to adverse environmental conditions. The increased ROS levels cause the severe destruction of cell organelles and their functions and ultimately may cause cell death. In order to maintain the balance of ROS at non-damaging levels, plants activate the antioxidant defense system [
11,
30,
31].
As noted in the introduction part, the possible toxic effect of anthelmintics in plants was evaluated in in vitro models using high concentrations of parent compounds. The simple phytotoxicity test using the “seed germination and early root length assay” revealed significant toxicity of IVM even at the lowest concentration of 50 nM tested on mustard seeds [
4]. On the other hand, neither ABZ nor ABZ-SO showed a toxic effect using this assay in mustard seeds [
1], and similarly, MOP showed no toxic effect in alfalfa seeds [
16]. Similar results were obtained by toxicity assay in
Arabidopsis thaliana cells where IVM decreased the viability of cells at 0.1 µM concentration, while ABZ decreased the viability at 10 µM and MOP had even inverse effect (increased viability) at 1 µM concentration (unpublished data). In a study on
Campanula rotundifolia cells, only moderate acute toxicity of ABZ was observed [
13].
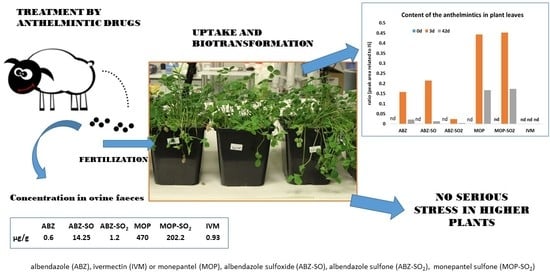
According to analyses quantifying the content of anthelmintics in ovine faeces, it is evident that, in real conditions, drugs are released to the environment in low doses and each drug in quite different amounts. Very low IVM concentrations with no metabolites were detected in ovine faeces [
4]. On the contrary, 17 times higher concentrations of ABZ and its metabolites, and over 700 times higher concentrations of MOP and its metabolites, when compared to IVM (see
Table 1), were detected in ovine faeces. The stress expressed in plants is a dose-dependent matter and very low doses of xenobiotics might even have an opposite effect, i.e., stimulate the plant metabolism and growth [
32]. Thus, we would like to know the effect of anthelmintics and their metabolites in real concentrations and conditions, i.e., in manure from sheep treated with anthelmintics in the course of regular deworming.
Our results showed no increased generation of H
2O
2 compared with the control during the whole period of cultivation after the application of faeces from treated sheep, not even in plants, where the uptake of the parent as well as metabolized (monitored) compounds of ABZ and MOP were confirmed from the third day, as the H
2O
2 is the most stable of other ROS and a key ROS product in reaction to several stress conditions [
29,
30]. In addition, no over accumulation of MDA, a product of the per-oxidized membrane lipids usually associated with stress response in plants [
33], was detected.
Proline is classified as an osmoprotectant, playing a protective role against the toxic effect of ROS, protein denaturation, and damage of cell structures. It is upregulated in plants under environmental stress [
12]. Moderate acute toxicity expressed by increased proline accumulation in alfalfa roots was observed shortly after the in vitro cultivated plants were transferred to a medium with MOP [
16]. By contrast, the IVM caused increased proline accumulation after long term exposure in in vitro cultivated plants of
Plantago lanceolata [
15]. In a study on in vitro cultivated
Medicago sativa, only ABZ (out of all tested anthelmintics) decreased proline content, and this trend was observed only in shoots (unpublished data). Although proline tends to accumulate early after stress exposure [
34], in our study simulating the real conditions, we observed moderate upregulation after long exposure in response to MOP only. MOP is considered less toxic according to our previous results. The proline accumulation can even increase by a hundred times under stressful conditions [
35]. Therefore, our results show an insignificant stress impact of anthelmintics on plant osmoprotection.
Regarding the enzymatic antioxidant defense system, we observed an increased CAT activity shortly after the exposure to IVM in
Plantago lanceolata in vitro regenerants and a decreasing tendency in APX activity following up to six days of cultivation in our previous study in in vitro conditions [
15]. The present study simulating the real exposure to anthelmintics showed activation of GST as a response to all anthelmintics, shortly after the application of faeces from treated sheep. GSTs are multifunctional enzymes and several reports state that GST enzymes are involved in the plant detoxification system [
31,
36]. Therefore, the increased activation of GST is logical, considering the fact that ABZ and MOP accumulation was detected in clover shoots immediately after the application of contaminated faeces. This observation also supports the presumption that IVM was accumulated as well, although not detected in shoots. GSTs catalyze the nucleophilic attack of the sulphur atom of glutathione [
31]. Glutathione is one of the non-enzymatic antioxidants, and thus is accumulated in response to oxidative stress conditions. Increased GST activity after three days was followed in our study by increased GR activity after 10 days, coincidently in response to all applied anthelmintics. The role of GR in the plant defense system is to maintain a high ratio of oxidized/reduced glutathione under various abiotic stresses. GR converts oxidized glutathione to reduced glutathione and thus helps to maintain the glutathione pool [
31,
37]. The major activity of GR is reported in chloroplast, where it plays an essential role in photoprotection, while the mitigation of oxidative stress is associated with GR activity in mitochondria and cytosol [
30,
31]. This indicates that the anthelmintics might suppress the light sensitivity of plants.
The moderate upregulation of GST and GR was observed also after long exposure of faeces from sheep treated with ABZ. The accumulation of ABZ and its metabolites in clover was detected in a continuous manner until almost degraded after six weeks. The response in increased GST and GR activity in a later phase of cultivation indicates that ABZ was gradually transported and degraded in shoot and resulted in continuous detoxification mechanism and over-accumulation of oxidized glutathione.
After three days, the application of faeces from sheep treated with IVM resulted in increased activity of not only GST, but also POX, APX, and SOD. Although the level of increase was not significant, except for SOD, this observation was surprising regarding the fact that no accumulation of IVM was detected in clover shoots three days after exposure. Actually, no accumulation of IVM was detected in clover shoots during the whole cultivation, or the content remains under the detection limit.
Stress-accrued ROS levels are in the first line usually reduced with SOD that converts superoxide radical (O
2−) to a product with a lower oxidation state, H
2O
2 and O
2. The H
2O
2 is further converted by CAT and POX to prevent the harmful formation of ROS [
10,
31]. The frontline strategy of SOD was proven by increased activity shortly after the application in response not only to IVM, considered as slightly phytotoxic, but also to MOP, considered non-phytotoxic, but accumulated more massively in plants at the beginning of co-cultivation. The in vitro studies suggesting that MOP might have a rather stimulating effect is proven by the decreased enzyme activity of POX and CAT after three weeks and which remained decreased after up to six weeks of cultivation. CAT activity was also decreased after long exposure to faeces from sheep treated with ABZ and IVM. Together with other peroxidases, CAT modulate H
2O
2 level within cells, while CAT scavenges the major part of H
2O
2, and the APX and GR finely regulate the balance in cellular H
2O
2 levels [
38]. In our study, no upregulation of CAT was observed, since no massive H
2O
2 overproduction was detected. On the contrary, CAT was downregulated after long exposure to all tested anthelmintics, and this reaction could be in response to slow decreasing H
2O
2 generation in plants, and also signify that anthelmintics do not induce photooxidative stress in plants [
30]. Similarly, no seriously increased APX activity was observed in our study. Only moderate upregulation was observed shortly after the application of faeces from sheep treated with IVM, where the complex enzymatic antioxidant defense system was activated. Normal APX activity seems logical due to the findings that APX is crucial for photoprotection and is also involved in plant defense to heat and drought stress and wounding [
30], and thus is not involved in xenobiotic detoxification machinery. Similarly, POX, which serves as a second line in defense system and scavenges the extracellular H
2O
2 [
31], was moderately upregulated shortly after exposure to IVM and ABZ. This may indicate ROS generation in extracellular space in response to anthelmintics. The POX was further downregulated after long exposure to MOP in comparison to plants that were not exposed to anthelmintics.