Light Intensity: The Role Player in Cucumber Response to Cold Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Condition
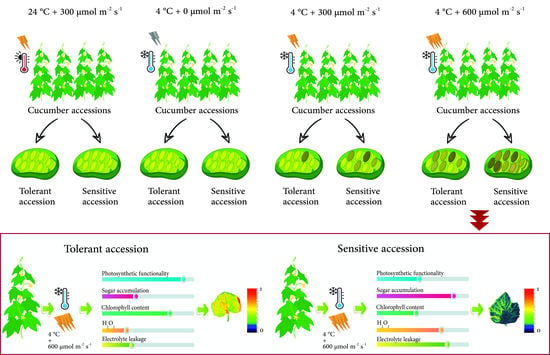
2.2. Light Treatments under Cold Condition
2.3. Chlorophyll Fluorescence Imaging and OJIP Test Measurements
2.4. Photosynthetic Pigments’ Measurements
2.5. Ascorbate Peroxidase (APX) Activity
2.6. Hydrogen Peroxide (H2O2) Content
2.7. Electrolyte Leakage (EL)
2.8. Water-Soluble Carbohydrates (WSC)
2.9. Statistical Analysis
3. Results
3.1. Biophysical Response of Cucumber Seedlings to Low Temperature Varies among Plants Exposed to Different PPFDs
3.2. Biophysical Response of Cucumber Seedlings to Low Temperature Varies among Plants Exposed to Different PPFDs
3.3. Low Temperature Decreased the Leaf Photosynthesis Pigment Contents in a PPFD-Dependent Manner
3.4. Water-Soluble Carbohydrate (WSC) Accumulates in Plants under Low Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, D.J.; Ort, D.R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef]
- Mashayekhi, K.; Jafari, S.; Shomali, A.; Mousavizadeh, S.J. Applying calcium carbide solution affect sex expression and increases yield of monoicous cucumber. J. Plant Physiol. Breed 2017, 7, 99–104. [Google Scholar]
- Shomali, A.; Aliniaeifard, S.; Didaran, F.; Lotfi, M.; Mohammadian, M.; Seif, M.; Strobel, W.R.; Sierka, E.; Kalaji, H.M. Synergistic effects of melatonin and Gamma-Aminobutyric Acid on protection of photosynthesis system in response to multiple abiotic stressors. Cells 2021, 10, 1631. [Google Scholar] [CrossRef]
- Shomali, A.; Aliniaeifard, S. Overview of signal transduction in plants under salt and drought stresses. In Salt and Drought Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 231–258. [Google Scholar]
- Aliniaeifard, S.; Shomali, A.; Seifikalhor, M.; Lastochkina, O. Calcium signaling in plants under drought. In Salt and Drought Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 259–298. [Google Scholar]
- Ibáñez, H.; Ballester, A.; Muñoz, R.; José Quiles, M. Chlororespiration and tolerance to drought, heat and high illumination. J. Plant Physiol. 2010, 167, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021, 48, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, M.; Aliniaeifard, S.; Shomali, A.; Didaran, F. Interaction of light intensity and CO2 concentration alters biomass partitioning in Chrysanthemum. J. Hortic. Res. 2021, 29. [Google Scholar] [CrossRef]
- Kozai, T. Why LED lighting for urban agriculture? In LED Lighting for Urban Agriculture; Springer: Singapore, 2016; pp. 3–18. ISBN 9789811018480. [Google Scholar]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H.; Yang, J. Photosynthetic properties and potentials for improvement of photosynthesis in pale green leaf rice under high light conditions. Front. Plant Sci. 2017, 8, 1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huner, N.P.A.; Öquist, G.; Hurry, V.M.; Krol, M.; Falk, S.; Griffith, M. Photosynthesis, photoinhibition and low temperature acclimation in cold tolerant plants. Photosynth. Res. 1993, 37, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Vranova, E.; Inzé, D.; Van Breusegem, F. Signal transduction during oxidative stress. J. Exp. Bot. 2002, 53, 1227–1236. [Google Scholar] [CrossRef] [Green Version]
- Demarsy, E.; Goldschmidt-Clermont, M.; Ulm, R. Coping with ‘dark sides of the sun’through photoreceptor signaling. Trends Plant Sci. 2018, 23, 260–271. [Google Scholar] [CrossRef]
- Janda, T.; Kissimon, J.; Szigeti, Z.; Veisz, O.; Páldi, E. Characterization of cold hardening in wheat using fluorescence induction parameters. J. Plant Physiol. 1994, 143, 385–388. [Google Scholar] [CrossRef]
- Gray, G.R.; Chauvin, L.-P.; Sarhan, F.; Huner, N.P.A. Cold acclimation and freezing tolerance (a complex interaction of light and temperature). Plant Physiol. 1997, 114, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanner, L.A.; Junttila, O. Cold-induced freezing tolerance in arabidopsis. Plant Physiol. 1999, 120, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Heldt, H.-W. Plant Biochemistry and Molecular Biology; Oxford University Press: Oxford, UK, 1997; ISBN 019850179X. [Google Scholar]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trend Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Hyldgaard, B.; Yu, X.; Rosenqvist, E.; Ugarte, R.M.; Yu, S.; Wu, Z.; Ottosen, C.O.; Zhao, T. Phenotyping of faba beans (Vicia faba L.) under cold and heat stresses using chlorophyll fluorescence. Euphytica 2018, 214, 68. [Google Scholar] [CrossRef]
- Moosavi-Nezhad, M.; Salehi, R.; Aliniaeifard, S.; Tsaniklidis, G.; Woltering, E.J.; Fanourakis, D.; Żuk-Gołaszewska, K.; Kalaji, H.M. Blue light improves photosynthetic performance during healing and acclimatization of grafted watermelon seedlings. Int. J. Mol. Sci. 2021, 22, 8043. [Google Scholar] [CrossRef]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Salami, S.A.; Shokrpour, M.; Pedersen, C.; Moosavi-Nezhad, M.; Wróbel, J.; Kalaji, H.M. Blue light improves photosynthetic performance and biomass partitioning toward harvestable organs in saffron (Crocus sativus L.). Cells 2021, 10, 1994. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, S.; Smillie, R.; Hardacre, A.; Eagles, H. Using chlorophyll fluorescence in vivo to measure the chilling tolerances of different populations of maize. Funct. Plant Biol. 1983, 10, 247. [Google Scholar] [CrossRef]
- Schapendonk, A.H.C.M.; Dolstra, O.; van Kooten, O. The use of chlorophyll fluorescence as a screening method for cold tolerance in maize. Photosynth. Res. 1989, 20, 235–247. [Google Scholar] [CrossRef]
- Dolstra, O.; Haalstra, S.R.; van der Putten, P.E.L.; Schapendonk, A.H.C.M. Genetic variation for resistance to low-temperature photoinhibition of photosynthesis in maize (Zea mays L.). Euphytica 1994, 80, 85–93. [Google Scholar] [CrossRef]
- Roden, J.S.; Egerton, J.J.G.; Ball, M.C. Effect of elevated [CO2] on photosynthesis and growth of snow gum (Eucalyptus pauciflora) seedlings during winter and spring. Aust. J. Plant Physiol. 1999, 26, 37–46. [Google Scholar] [CrossRef]
- Walker, D. The Use of the Oxygen Electrode and Fluorescence Probes in Simple Measurements of Photosynthesis; Citeseer: Pennsylvania, PA, USA, 1987; ISBN 1870232003. [Google Scholar]
- Roháček, K. Chlorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB Plants 2018, 10, ply052. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as early indicators of light stress in barley. J. Photochem. Photobiol. B Biol. 2012, 112, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Lazár, D.; Kromdijk, J. Govindjee Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Kalhor, M.S.; Aliniaeifard, S.; Seif, M.; Asayesh, E.J.; Bernard, F.; Hassani, B.; Li, T. Enhanced salt tolerance and photosynthetic performance: Implication of γ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol. Biochem. 2018, 130, 157–172. [Google Scholar] [CrossRef]
- Dai, Y.; Yuan, L.; Zhang, S.; Wang, J.; Xie, S.; Zhao, M.; Chen, G.; Sun, R.; Wang, C. Comprehensive evaluation for cold tolerance in wucai (brassica campestris L.) by the performance index on an absorption basis (PIabs). Agronomy 2019, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Estaji, A.; Kalaji, H.M.; Karimi, H.R.; Roosta, H.R.; Moosavi-Nezhad, S.M. How glycine betaine induces tolerance of cucumber plants to salinity stress? Photosynthetica 2019, 57, 753–761. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, M.H.; Goltsev, V.N.; Żuk-Golaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781498764506. [Google Scholar]
- Antal, T.; Rubin, A. In vivo analysis of chlorophyll a fluorescence induction. Photosynth. Res. 2008, 96, 217–226. [Google Scholar] [CrossRef]
- Szalai, G.; Pap, M.; Janda, T. Light-induced frost tolerance differs in winter and spring wheat plants. J. Plant Physiol. 2009, 166, 1826–1831. [Google Scholar] [CrossRef]
- Prerostova, S.; Černý, M.; Dobrev, P.I.; Motyka, V.; Hluskova, L.; Zupkova, B.; Gaudinova, A.; Knirsch, V.; Janda, T.; Brzobohatý, B.; et al. Light regulates the cytokinin-dependent cold stress responses in Arabidopsis. Front. Plant Sci. 2021, 11, 2293. [Google Scholar] [CrossRef] [PubMed]
- Janda, T.; Majláth, I.; Szalai, G. Interaction of temperature and light in the development of freezing tolerance in plants. J. Plant Growth Regul. 2014, 33, 460–469. [Google Scholar] [CrossRef] [Green Version]
- Soitamo, A.J.; Piippo, M.; Allahverdiyeva, Y.; Battchikova, N.; Aro, E.M. Light has a specific role in modulating Arabidopsis gene expression at low temperature. BMC Plant Biol. 2008, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Malcolm Matamoros, P.; Van Meeteren, U. Stomatal malfunctioning under low Vapor Pressure Deficit (VPD) conditions: Induced by alterations in stomatal morphology and leaf anatomy or in the ABA signaling. Physiol. Plant. 2014, 152, 688–699. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Van Meeteren, U. Natural variation in stomatal response to closing stimuli among Arabidopsis thaliana accessions after exposure to low VPD as a tool to recognize the mechanism of disturbed stomatal functioning. J. Exp. Bot. 2014, 65, 6529–6542. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Shiferaw, B.; Baker, D.A. An evaluation of drought screening techniques for Eragrostis tef. Trop. Sci. 1996, 36, 74–85. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Ayyaz, A.; Amir, M.; Umer, S.; Iqbal, M.; Bano, H.; Gul, H.S.; Noor, Y.; kanwal, A.; khalid, A.; Javed, M.; et al. Melatonin induced changes in photosynthetic efficiency as probed by OJIP associated with improved chromium stress tolerance in canola (Brassica napus L.). Heliyon 2020, 6, e04364. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta-Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [Green Version]
- Raju, S.K.K.; Barnes, A.C.; Schnable, J.C.; Roston, R.L. Low-temperature tolerance in land plants: Are transcript and membrane responses conserved? Plant Sci. 2018, 276, 73–86. [Google Scholar] [CrossRef]
- Colom, M.R.; Pini Prato, E.; Giannini, R. Chlorophyll fluorescence and photosynthetic response to light in 1-year-old needles during spring and early summer in Pinus leucodermis. Trees-Struct. Funct. 2003, 17, 207–210. [Google Scholar] [CrossRef]
- Fracheboud, Y.; Haldimann, P.; Leipner, J.; Stamp, P. Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). J. Exp. Bot. 1999, 50, 1533–1540. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Noedoost, F.; Geuns, J.; Djalovic, I.; Siddique, K.H.M. Effect of cold stress on photosynthetic traits, carbohydrates, morphology, and anatomy in nine cultivars of Stevia rebaudiana. Front. Plant Sci. 2018, 9, 1430. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-J.; Chang, W.; Huang, W.; Zhang, S.-B.; Hu, H. The effects of chilling-light stress on photosystems I and II in three Paphiopedilum species. Bot. Stud. 2017, 58, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allakhverdiev, S.I.; Murata, N. Salt stress inhibits photosystems II and I in cyanobacteria. Photosynth. Res. 2008, 98, 529–539. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Corrigendum to: Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2020, 71, 1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A.; Nicola, S. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiol. Biochem. 2016, 106, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation; Taylor & Francis: London, UK, 2000; pp. 443–480. ISBN 0748408215. [Google Scholar]
- Foyer, C.H.; Neukermans, J.; Queval, G.; Noctor, G.; Harbinson, J. Photosynthetic control of electron transport and the regulation of gene expression. J. Exp. Bot. 2012, 63, 1637–1661. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, V.M.; Soengas, P.; Alonso-Villaverde, V.; Sotelo, T.; Cartea, M.E.; Velasco, P. Effect of temperature stress on the early vegetative development of Brassica oleracea L. BMC Plant Biol. 2015, 15, 145. [Google Scholar] [CrossRef] [Green Version]
- van Heerden, P.D.R.; Swanepoel, J.W.; Krüger, G.H.J. Modulation of photosynthesis by drought in two desert scrub species exhibiting C3-mode CO2 assimilation. Environ. Exp. Bot. 2007, 61, 124–136. [Google Scholar] [CrossRef]
- Perveen, S.; Shahbaz, M.; Ashraf, M. Regulation in gas exchange and quantum yield of photosystem II (PSII) in salt-stressed and non-stressed wheat plants raised from seed treated with triacontanol. Pak. J. Bot 2010, 42, 3073–3081. [Google Scholar]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Steffens, B. The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front. Plant Sci. 2014, 5, 685. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [Green Version]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Response of oxidative metabolism in watermelon plants subjected to cold stress. Funct. Plant Biol. 2002, 29, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Bonnecarrère, V.; Borsani, O.; Díaz, P.; Capdevielle, F.; Blanco, P.; Monza, J. Response to photoxidative stress induced by cold in japonica rice is genotype dependent. Plant Sci. 2011, 180, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Scandalios, J.G. The rise of ROS. Trends Biochem. Sci. 2002, 27, 483–486. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wu, J.; Zheng, X.; Zheng, S.; Sun, X.; Qiu, Q.; Lu, T. Gene knockout study reveals that cytosolic ascorbate peroxidase 2 (OsAPX2) plays a critical role in growth and reproduction in rice under drought, salt and cold stresses. PLoS ONE 2013, 8, e57472. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Duan, X.; Jiang, Y.; Zhang, P. Increased expression of native cytosolic Cu/Zn superoxide dismutase and ascorbate peroxidase improves tolerance to oxidative and chilling stresses in cassava (Manihot esculenta Crantz). BMC Plant Biol. 2014, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Chai, H.; Yin, H.; Yang, M.; Hu, G.; Guo, M.; Yi, R.; Zhang, P. Full-length transcriptome sequencing reveals the low-temperature-tolerance mechanism of Medicago falcata roots. BMC Plant Biol. 2019, 19, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.E.; Kim, S.; Han, T.J.; Lee, J.S.; Chang, S.C. ABA and polyamines act independently in primary leaves of cold-stressed tomato (Lycopersicon esculentum). Physiol. Plant. 2002, 115, 370–376. [Google Scholar] [CrossRef]
- Nasibi, F.; Manouchehri Kalantari, K.; Manzari Tavakoli, Z. Effects of hydrogen sulfide on cold-induced oxidative damage in Cucumis sativus L. Introduction. Int. J. Hortic. Sci. Technol. 2020, 7, 199–211. [Google Scholar]
- Shahryar, N.; Maali-Amiri, R. Metabolic acclimation of tetraploid and hexaploid wheats by cold stress-induced carbohydrate accumulation. J. Plant Physiol. 2016, 204, 44–53. [Google Scholar] [CrossRef]
- Améglio, T.; Cochard, H.; Ewers, F.W. Stem diameter variations and cold hardiness in walnut trees. J. Exp. Bot. 2001, 52, 2135–2142. [Google Scholar] [CrossRef]
- Wang, K.; Shao, X.; Gong, Y.; Zhu, Y.; Wang, H.; Zhang, X.; Yu, D.; Yu, F.; Qiu, Z.; Lu, H. The metabolism of soluble carbohydrates related to chilling injury in peach fruit exposed to cold stress. Postharvest Biol. Technol. 2013, 86, 53–61. [Google Scholar] [CrossRef]
- Yuanyuan, M.; Yali, Z.; Jiang, L.; Hongbo, S. Roles of plant soluble sugars and their responses to plant cold stress. Afr. J. Biotechnol. 2009, 8, 2004–2010. [Google Scholar]
- Adams, W.W.; Muller, O.; Cohu, C.M.; Demmig-Adams, B. May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynth. Res. 2013, 117, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Heyer, A.G.; Mishra, K.B. Chlorophyll fluorescence emission can screen cold tolerance of cold acclimated Arabidopsis thaliana accessions. Plant Methods 2014, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Jiang, F.; Wang, X.; Zang, Y.; Wu, Z. Comprehensive evaluation and screening for chilling-tolerance in tomato lines at the seedling stage. Euphytica 2015, 205, 569–584. [Google Scholar] [CrossRef]
- Aazami, M.A.; Asghari-Aruq, M.; Hassanpouraghdam, M.B.; Ercisli, S.; Baron, M.; Sochor, J. Low temperature stress mediates the antioxidants pool and chlorophyll fluorescence in Vitis vinifera L. Cultivars. Plants 2021, 10, 1877. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Wang, J.; Yu, X.; He, C.; Li, Y. Substrate application of 5-aminolevulinic acid enhanced low-temperature and weak-light stress tolerance in cucumber (Cucumis sativus L.). Agronomy 2020, 10, 472. [Google Scholar] [CrossRef] [Green Version]








| Abbreviation | Equation | Definition |
|---|---|---|
| FV/F0 | ϕ P0/(1 − ϕ P0) | Maximum efficiency of the water diffusion reaction on the donor side of PSII |
| FV/FM | TR0/ABS = [1 − (F0/FM)] | Relative maximal variable fluorescence |
| FM/F0 | Structural damage to PSII | |
| ABS/RC | M0 (1/VJ)(1/ϕP0) | Light absorbance flux for PSII antenna chlorophylls per reaction center |
| TR0/RC | M0 (1/VJ) | Trapped energy flux per reaction center |
| ET0/RC | M0 (1/VJ) ψ0 | Electron transport flux per reaction center |
| DI0/RC | (ABS/RC) − (TR0/RC) | Energy flux not intercepted by an RC, dissipated in the form of heat, fluorescence, or transfer to other systems, at time t = 0 |
| PIabs | (RC/ABS) × (ϕP0/(1 − ϕP0)) × (ψ0/(1 − ψ0)) | Performance index per absorbed light |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashrostaghi, T.; Aliniaeifard, S.; Shomali, A.; Azizinia, S.; Abbasi Koohpalekani, J.; Moosavi-Nezhad, M.; Gruda, N.S. Light Intensity: The Role Player in Cucumber Response to Cold Stress. Agronomy 2022, 12, 201. https://doi.org/10.3390/agronomy12010201
Ashrostaghi T, Aliniaeifard S, Shomali A, Azizinia S, Abbasi Koohpalekani J, Moosavi-Nezhad M, Gruda NS. Light Intensity: The Role Player in Cucumber Response to Cold Stress. Agronomy. 2022; 12(1):201. https://doi.org/10.3390/agronomy12010201
Chicago/Turabian StyleAshrostaghi, Tahereh, Sasan Aliniaeifard, Aida Shomali, Shiva Azizinia, Jahangir Abbasi Koohpalekani, Moein Moosavi-Nezhad, and Nazim S. Gruda. 2022. "Light Intensity: The Role Player in Cucumber Response to Cold Stress" Agronomy 12, no. 1: 201. https://doi.org/10.3390/agronomy12010201
APA StyleAshrostaghi, T., Aliniaeifard, S., Shomali, A., Azizinia, S., Abbasi Koohpalekani, J., Moosavi-Nezhad, M., & Gruda, N. S. (2022). Light Intensity: The Role Player in Cucumber Response to Cold Stress. Agronomy, 12(1), 201. https://doi.org/10.3390/agronomy12010201