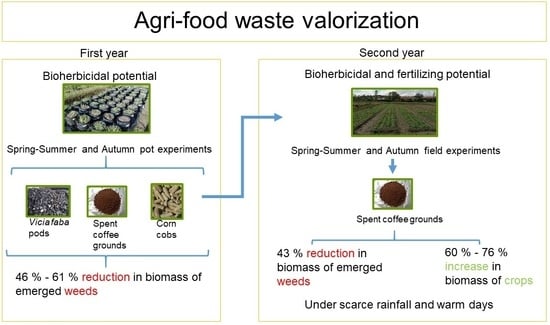
Agri-Food Waste as a Method for Weed Control and Soil Amendment in Crops
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluated Waste
2.2. Waste Extracts for Pot Experiments
2.3. Crop Species for Field Evaluation
2.4. Experimental Set-Up
2.4.1. Pot Trials
Experiment 1—Bioherbicidal Effect of Different Waste on Emerging Spring–Summer Weeds
Experiment 2—Bioherbicidal Effect of Different Waste on Emerging Autumn Weeds
2.4.2. Field Evaluation
Experiment 3—Evaluation of Agri-Food Waste Effects on Spring–Summer Crops and Associated Weeds
Experiment 4—Evaluation of Agri-Food Waste Effect on Autumn Crops and Associated Weeds
2.5. Statistical Analyses
3. Results
3.1. Bioherbicidal Effect on Emerging Common Weeds in Pots
3.2. Waste Performance in Field Crops
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cycon, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef] [PubMed]
- De Corato, U. Towards new soil management strategies for improving soil quality and ecosystem services in sustainable agriculture: Editorial overview. Sustainability 2020, 12, 9398. [Google Scholar] [CrossRef]
- Khan, M.A.; Costa, F.B.; Fenton, O.; Jordan, P.; Fennell, C.; Mellander, P.E. Using a multi-dimensional approach for catchment scale herbicide pollution assessments. Sci. Total Environ. 2020, 747, 141232. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Heap, I. Evolution of weed resistance to herbicides: What have we learned after 70 years? In Biology, Physiology and Molecular Biology of Weeds; Jugulam, M., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 63–86. [Google Scholar]
- Green, J.M.; Owen, M.D.K. Herbicide-resistant crops: Utilities and limitations for herbicide-resistant weed management. J. Agric. Food Chem. 2011, 59, 5819–5829. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Babalola, O.O. The multifaceted plant-beneficial rhizobacteria toward agricultural sustainability. Plant Protect. Sci. 2021, 57, 95–111. [Google Scholar]
- European Commission (EC). Regulation No 834/2007. Official Journal of the European Union on Organic Production and Labeling of Organic Products and Repealing Regulation (EEC); No 2092/91. OJL 189, 20.7.2007; European Commission (EC): Brussels, Belgium, 2007; pp. 1–23. [Google Scholar]
- European Commission (EC). Organic Farming in the EU. A Fast Growing Sector; EU Agricultural Markets Briefs; European Commission (EC): Brussels, Belgium, 2019. [Google Scholar]
- European Commission (EC). Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System; Communication No 381/2020. 20.5.2020; European Commission (EC): Brussels, Belgium, 2020; pp. 1–20. [Google Scholar]
- Semida, W.M.; Beheiry, H.R.; Sétamou, M.; Simpson, C.R.; Abd El-Mageed, T.A.; Rady, M.M.; Nelson, S.D. Biochar implications for sustainable agriculture and environment: A review. S. Afr. J. Bot. 2019, 127, 333–347. [Google Scholar] [CrossRef]
- Lorenzo, P.; Reboredo-Durán, J.; Muñoz, L.; Freitas, H.; González, L. Herbicidal properties of the commercial formulation of methyl cinnamate, a natural compound in the invasive silver wattle (Acacia dealbata). Weed Sci. 2020, 68, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Puig, C.G.; Álvarez-Iglesias, L.; Reigosa, M.J.; Pedrol, N. Eucalyptus globulus leaves incorporated as green manure for weed control in maize. Weed Sci. 2013, 61, 154–161. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; Puig, C.G.; Pedrol, N.; Freitas, H.; Rodríguez-Echeverría, S.; Lorenzo, P. Exploring the use of residues from the invasive Acacia sp. for weed control. Renew. Agric. Food Syst. 2020, 35, 26–37. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E.; Romagni, J.G.; Rimando, A.M. Natural products as sources of herbicides: Current status and future trends. Weed Res. 2000, 40, 99–111. [Google Scholar] [CrossRef]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Kadoglidou, K.; Kalaitzidis, A.; Stavrakoudis, D.; Mygdalia, A.; Katsantonis, D. A novel compost for rice cultivation developed by rice industrial by-products to serve circular economy. Agronomy 2019, 9, 553. [Google Scholar] [CrossRef] [Green Version]
- Pellejero, G.; Palacios, J.; Vela, E.; Gajardo, O.; Albrecht, L.; Aschkar, G.; Chrorolque, A.; García-Navarro, F.J.; Jiménez-Ballesta, R. Effect of the application of compost as an organic fertilizer on a tomato crop (Solanum lycopersicum L.) produced in the field in the Lower Valley of the Río Negro (Argentina). Int. J. Recycl. Org. Waste Agric. 2021, 10, 145–155. [Google Scholar]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Chen, C.; Wang, D.; Arthur, E.; Zhang, Z.; Guo, Z.; Peng, X.; Mooney, S.J. Effect of long-term organic amendments on the full-range soil water retention characteristics of a Vertisol. Soil Till. Res. 2020, 202, 104663. [Google Scholar] [CrossRef]
- Campos, E.V.; Proença, P.L.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef] [Green Version]
- European Commission (EC). Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2008/98/EC on Waste; OJL 150, 14.6.2018; European Commission (EC): Brussels, Belgium, 2018; pp. 109–140. [Google Scholar]
- Oliveira, T.; Dias, R.R. Tendências Económico-Sociais No Consumo de Café em Portugal Para. 2021. Available online: https://www.academia.edu/16593649/O_Cafe_Perspectivas_e_Tende_ncias_Sociais_em_Portugal (accessed on 30 September 2021).
- Caldeira, D.C.A. Valorização da Borra de Café: Otimização da Produção de Biodiesel por Catálise Enzimática. Ph.D. Thesis, Instituto Superior de Engenharia do Porto, Porto, Portugal, 2015. [Google Scholar]
- AMPROMIS. Associaçião Nacional dos Produtores de Milho e Sorgo. 2021. Available online: https://www.anpromis.pt/ (accessed on 25 August 2021).
- Pinto, J.; Cruz, D.; Paiva, A.; Pereira, S.; Tavares, P.; Fernandes, L.; Varum, H. Characterization of corn cob as a possible raw building material. Constr. Build. Mater. 2012, 34, 28–33. [Google Scholar] [CrossRef]
- Ramos, A.; Briga-Sá, A.; Pereira, S.; Correia, M.; Pinto, J.; Bentes, I.; Teixeira, C.A. Thermal performance and life cycle assessment of corn cob particleboards. J. Build. Eng. 2021, 44, 102998. [Google Scholar] [CrossRef]
- Viana, R.L.S.; Fidelis, G.P.; Medeiros, M.J.C.; Morgano, M.A.; Alves, M.G.C.F.; Passero, L.F.D.; Pontes, D.L.; Theodoro, R.C.; Arantes, T.D.; Sabry, D.A.; et al. Green synthesis of antileishmanial and antifungal silver nanoparticles using corn cob xylan as a reducing and stabilizing agent. Biomolecules 2020, 10, 1235. [Google Scholar] [CrossRef]
- Stylianou, M.; Agapiou, A.; Omirou, M.; Vyrides, I.; Ioannides, I.M.; Maratheftis, G.; Fasoula, D. Converting environmental risks to benefits by using spent coffee grounds (SCG) as a valuable resource. Environ. Sci. Pollut. R. 2018, 25, 35776–35790. [Google Scholar] [CrossRef] [PubMed]
- McNutt, J.; He, Q. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Yamane, K.; Kono, M.; Fukunaga, T.; Iwai, K.; Sekine, R.; Watanabe, Y.; Iijima, M. Field evaluation of coffee grounds application for crop growth enhancement, weed control, and soil improvement. Plant Prod. Sci. 2014, 17, 93–102. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Pastoriza, S.; Rufián-Henares, J.A.; Párraga, J.; Martín-García, J.M.; Delgado, G. Impact of spent coffee grounds as organic amendment on soil fertility and lettuce growth in two Mediterranean agricultural soils. Arch. Agron. Soil Sci. 2018, 64, 790–804. [Google Scholar] [CrossRef]
- Hardgrove, S.J.; Livesley, S.J. Applying spent coffee grounds directly to urban agriculture soils greatly reduces plant growth. Urban For. Urban Green. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, J.A.; Ramalhosa, E.; Casal, S.; Baptista, P. Effect of fresh and composted spent coffee grounds on lettuce growth, photosynthetic pigments and mineral composition. In VII Congreso Ibérico de Agroingeniería y Ciencias Horticolas; Téllez, A., Masaguer, F., Sancho, I., Robinson, M., Altisent, M., Ballesteros, F., Strap, E., Eds.; SECH e SEAgIng: Madrid, Spain, 2014; pp. 1–5. [Google Scholar]
- Batish, D.R.; Singh, H.P.; Kaur, M.; Kohli, R.K.; Yadav, S.S. Caffeine affects adventitious rooting and causes biochemical changes in the hypocotyl cuttings of mung bean (Phaseolus aureus Roxb.). Acta Physiol. Plant. 2008, 30, 401–405. [Google Scholar] [CrossRef]
- Álvarez-Iglesias, L.; Puig, C.G.; Revilla, P.; Reigosa, M.J.; Pedrol, N. Faba bean as green manure for field weed control in maize. Weed Res. 2018, 58, 437–449. [Google Scholar] [CrossRef]
- Al-Chammaa, M.; Al-Ain, F.; Kurdali, F. Growth, nitrogen and phosphorus uptake of sorghum plants as affected by green manuring with pea or faba bean shell pod wastes using N. Open Agric. J. 2019, 13, 133–145. [Google Scholar] [CrossRef]
- Roshan-Bakhsh, A.; Pourjam, E.; Ayyari, M.; Pedram, M. Biocontrol properties of some agricultural waste extracts on three nematode species in in vitro and in vivo conditions. Nematology 2019, 21, 837–846. [Google Scholar] [CrossRef]
- Hernández, M.; Ventura, J.; Castro, C.; Boone, V.; Rojas, R.; Ascacio-Valdés, J.; Martínez-Ávila, G. Uplc-esi-qtof-ms2-based identification and antioxidant activity assessment of phenolic compounds from red corn cob (Zea mays L.). Molecules 2018, 23, 1425. [Google Scholar] [CrossRef] [Green Version]
- Gullón, P.; Eibes, G.; Lorenzo, J.M.; Pérez-Rodríguez, N.; Lú-Chau, T.A.; Gullón, B. Green sustainable process to revalorize purple corn cobs within a biorefinery frame: Co-production of bioactive extracts. Sci. Total Environ. 2020, 709, 136236. [Google Scholar] [CrossRef] [PubMed]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Guilherme, R.; Reboredo, F.; Guerra, M.; Ressurreição, S.; Alvarenga, N. Elemental composition and some nutritional parameters of sweet pepper from organic and conventional agriculture. Plants 2020, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Nadeem, M.A.; Tanveer, A.; Ali, H.H.; Farooq, N. Role of allelopathic crop mulches and reduced doses of tank-mixed herbicides in managing herbicide-resistant Phalaris minor in wheat. Crop Prot. 2018, 110, 245–250. [Google Scholar] [CrossRef]
- Farooq, M.; Nawaz, A.; Ahmad, E.; Nadeem, F.; Hussain, M.; Siddique, K.H. Using sorghum to suppress weeds in dry seeded aerobic and puddled transplanted rice. Field Crop. Res. 2017, 214, 211–218. [Google Scholar] [CrossRef]
- Magalhães, M.C.; Cameira, M.C.; Pato, R.L.; Santos, F.; Bandeira, J. Biomassa florestal residual: Efeitos da sua remoção na qualidade do solo. Rev. Ciências Agrárias 2011, 34, 205–217. [Google Scholar]
- Almeida, D. Manual de Culturas Hortícolas; Coleção: Guias Práticos; Presença: Lisboa, Portugal, 2006; Volume II, p. 400. [Google Scholar]
- Ali, A.M.; Thind, H.S. A framework for refining nitrogen management in dry direct-seeded rice using GreenSeeker™ optical sensor. Comput. Electron. Agric. 2015, 110, 114–120. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Álvarez-Iglesias, L. Vicia faba L. for Weed Control: From Biochemical Evidences to Field Application. Ph.D. Thesis, Universidade de Vigo, Vigo, Spain, 2015. [Google Scholar]
- Osunde, M.O.; Olayinka, A.; Fashina, C.D.; Torimiro, N. Effect of carbon-nitrogen ratios of lignocellulosic substrates on the yield of mushroom (Pleurotus pulmonarius). OALib. J. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Miranda, J.; Costa, L.M.D.; Ruiz, H.A.; Einloft, R. Composição química da solução de solo sob diferentes coberturas vegetais e análise de carbono orgânico solúvel no deflúvio de pequenos cursos de água. Rev. Bras. Cienc. Solo 2006, 30, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Urbaniak, M.; Gągała, I.; Szewczyk, M.; Bednarek, A. Leaching of PCBs and nutrients from soil fertilized with municipal sewage sludge. Bull. Environ. Contam. Tox. 2016, 97, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Xylia, P.; Petropoulos, S.; Tzortzakis, N. The use of spent coffee grounds in growing media for the production of Brassica seedlings in nurseries. Environ. Sci. Pollut. Res. 2021, 28, 24279–24290. [Google Scholar] [CrossRef] [PubMed]
- Peerzada, A.M. Biology, agricultural impact, and management of Cyperus rotundus L.: The world’s most tenacious weed. Acta Physiol. Plant. 2017, 39, 270. [Google Scholar] [CrossRef]
- Riemens, M.M.; van der Weide, R.Y.; Runia, W.T. Nutsedge, Biology and Control of Cyperus rotundus and Cyperus esculentus, Review of a Literature Survey; Plant Research International B.V.: Wageningen, The Netherlands, 2008. [Google Scholar]




| Treatment | Abbreviation | Dose |
|---|---|---|
| River water/no waste (negative control) (L ha−1) | C | 45,418.8 |
| Vicia faba pod extract (L ha−1) | FE | 45,418.8 |
| Urtica dioca extract (L ha−1) | UE | 45,418.8 |
| Vicia faba pod waste (Mg ha−1) | F | 28 |
| Urtica dioca waste (Mg ha−1) | U | 9 |
| Herbicide (positive control) (L ha−1) | H | 3 |
| Treatment | Abbreviation | Dose |
|---|---|---|
| River water/no waste (negative control) (L ha−1) | C | 45,418.8 |
| Vicia faba pod extract (L ha−1) | FE | 45,418.8 |
| Urtica dioca extract (L ha−1) | UE | 45,418.8 |
| Vicia faba pod waste (Mg ha−1) | F | 9 |
| Urtica dioca waste (Mg ha−1) | U | 9 |
| Spent coffee grounds waste (Mg ha−1) | CG | 28 |
| Corn cob waste (Mg ha−1) | CC | 9 |
| Herbicide (positive control) (L ha−1) | H | 3 |
| Treatment | pH | Organic Matter (%) | N (%) | P2O5 (mg kg−1) | K2O (mg kg−1) |
|---|---|---|---|---|---|
| River water/no waste (negative control) | 6.06 ± 0.17 c | 2.70 ± 0.28 c | 0.17 ± 0.02 b | 422 ± 120 | 390 ± 160 |
| Vicia faba pod extract | 6.40 ± 0.17 ab | 2.81 ± 0.28 bc | 0.17 ± 0.02 b | 358 ± 120 | 413 ± 160 |
| Urtica dioca extract | 6.44 ± 0.17 ab | 2.80 ± 0.28 bc | 0.17 ± 0.02 b | 298 ± 120 | 346 ± 160 |
| Vicia faba pod waste | 6.41 ± 0.17 ab | 3.15 ± 0.28 b | 0.19 ± 0.02 ab | 350 ± 120 | 576 ± 160 |
| Urtica dioca waste | 6.57 ± 0.17 a | 3.12 ± 0.28 bc | 0.19 ± 0.02 ab | 390 ± 120 | 442 ± 160 |
| Spent coffee grounds waste | 6.28 ± 0.17 bc | 3.86 ± 0.28 a | 0.22 ± 0.02 a | 309 ± 120 | 316 ± 160 |
| Corn cob waste | 6.46 ± 0.17 ab | 2.91 ± 0.28 bc | 0.16 ± 0.02 b | 255 ± 120 | 373 ± 160 |
| Herbicide (positive control) | 6.32 ± 0.17 abc | 2.73 ± 0.28 bc | 0.17 ± 0.02 b | 388 ± 120 | 333 ± 160 |
| p | <0.001 | <0.001 | <0.001 | 0.116 | 0.052 |
| Season | Source of Variation | Source Level | pH | Organic Matter (%) | N (%) | P2O5 (mg kg−1) | K2O (mg kg−1) |
|---|---|---|---|---|---|---|---|
| Spring–Summer | Treatment (Tr) | C | 6.79 ± 0.17 | 1.89 ± 0.30 | 0.123 ± 0.02 | 191 ± 120.0 | 227 ± 63.0 |
| F | 6.90 ± 0.17 | 1.91 ± 0.30 | 0.129 ± 0.02 | 251 ± 120.0 | 265 ± 63.0 | ||
| CG | 6.89 ± 0.17 | 2.06 ± 0.30 | 0.144 ± 0.02 | 218 ± 120.0 | 267 ± 63.0 | ||
| CC | 6.96 ± 0.17 | 1.88 ± 0.29 | 0.130 ± 0.02 | 215 ± 120.1 | 295 ± 63.0 | ||
| p | 0.285 | 0.643 | 0.137 | 0.817 | 0.259 | ||
| Time (t) | W4 | 6.92 ± 0.11 | 2.07 ± 0.19 a | 0.133 ± 0.01 | 246 ± 75.0 | 309 ± 40.0 a | |
| W8 | 6.84 ± 0.11 | 1.80 ± 0.18 b | 0.129 ± 0.01 | 192 ± 75.0 | 218 ± 39.0 b | ||
| p | 0.207 | 0.021 | 0.559 | 0.196 | 0.001 | ||
| Tr × t | C W4 | 6.83 ± 0.2 | 1.80 ± 0.46 | 0.123 ± 0.03 | 191 ± 188.0 | 271 ± 98.6 | |
| F W4 | 6.95 ± 0.26 | 2.15 ± 0.46 | 0.139 ± 0.03 | 306 ± 188.2 | 335 ± 98.6 | ||
| CG W4 | 6.95 ± 0.26 | 2.23 ± 0.47 | 0.141 ± 0.03 | 234 ± 188.2 | 298 ± 98.9 | ||
| CC W4 | 6.97 ± 0.27 | 2.10 ± 0.46 | 0.130 ± 0.03 | 252 ± 188.2 | 334 ± 99.0 | ||
| C W8 | 6.75 ± 0.26 | 1.99 ± 0.47 | 0.122 ± 0.03 | 190 ± 188.0 | 184 ± 98.8 | ||
| F W8 | 6.85 ± 0.26 | 1.66 ± 0.46 | 0.119 ± 0.03 | 197 ± 188.0 | 196 ± 98.5 | ||
| CG W8 | 6.83 ± 0.27 | 1.89 ± 0.46 | 0.147 ± 0.03 | 202 ± 188.0 | 237 ± 99.0 | ||
| CC W8 | 6.95 ± 0.2 | 1.66 ± 0.46 | 0.130 ± 0.03 | 178 ± 188.5 | 256 ± 98.9 | ||
| p | 0.950 | 0.139 | 0.528 | 0.884 | 0.670 | ||
| Autumn | Treatment | C | 6.41 ± 0.17 a | 1.92 ± 0.21 ab | 0.146 ± 0.01 ab | 244 ± 69.0 a | 304 ± 74.0 a |
| F | 6.15 ± 0.18 b | 1.82 ± 0.21 b | 0.126 ± 0.01 c | 122 ± 34.2 b | 191 ± 74.0 b | ||
| CG | 6.39 ± 0.18 ab | 2.16 ± 0.21 a | 0.161 ± 0.01 a | 299 ± 103.0 a | 247 ± 74.0 ab | ||
| CC | 6.36 ± 0.18 ab | 2.04 ± 0.21 ab | 0.141 ± 0.01 bc | 186 ± 52.0 ab | 203 ± 74.0 ab | ||
| p | 0.032 | 0.023 | <0.001 | <0.001 | 0.023 | ||
| Time | W4 | 6.29 ± 0.11 | 2.01 ± 0.13 | 0.149 ± 0.01 a | 264 ± 54.0 a | 248 ± 46.0 | |
| W8 | 6.36 ± 0.11 | 1.95 ± 0.13 | 0.138 ± 0.01 b | 161 ± 31.0 b | 224 ± 46.0 | ||
| p | 0.263 | 0.438 | 0.036 | 0.009 | 0.396 | ||
| Tr × t | C W4 | 6.36 ± 0.28 | 1.97 ± 0.33 | 0.158 ± 0.02 | 228 ± 99.0 bc | 313 ±116.0 | |
| F W4 | 6.06 ± 0.27 | 1.81 ± 0.33 | 0.129 ± 0.02 | 133 ± 57.6 bcd | 196 ± 116.0 | ||
| CG W4 | 6.36 ± 0.27 | 2.27 ± 0.33 | 0.167 ± 0.02 | 512 ± 221.5 a | 261 ± 115.4 | ||
| CC W4 | 6.38 ± 0.27 | 2.00 ± 0.33 | 0.142 ± 0.02 | 185 ± 80.1 bc | 221 ± 115.0 | ||
| C W8 | 6.46 ± 0.28 | 1.87 ± 0.33 | 0.135 ± 0.02 | 261 ± 112.8 ab | 294 ± 116.0 | ||
| F W8 | 6.24 ± 0.28 | 1.82 ± 0.33 | 0.123 ± 0.02 | 111 ± 48.0 cd | 185 ± 115.2 | ||
| CG W8 | 6.42 ± 0.28 | 2.05 ± 0.33 | 0.154 ± 0.02 | 85 ± 36.8 d | 233 ± 116.0 | ||
| CC W8 | 6.33 ± 0.28 | 2.08 ± 0.33 | 0.140 ± 0.02 | 187 ± 81.6 bc | 185 ± 115.7 | ||
| p | 0.677 | 0.537 | 0.448 | <0.001 | 0.989 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo, P.; Guilherme, R.; Barbosa, S.; Ferreira, A.J.D.; Galhano, C. Agri-Food Waste as a Method for Weed Control and Soil Amendment in Crops. Agronomy 2022, 12, 1184. https://doi.org/10.3390/agronomy12051184
Lorenzo P, Guilherme R, Barbosa S, Ferreira AJD, Galhano C. Agri-Food Waste as a Method for Weed Control and Soil Amendment in Crops. Agronomy. 2022; 12(5):1184. https://doi.org/10.3390/agronomy12051184
Chicago/Turabian StyleLorenzo, Paula, Rosa Guilherme, Sara Barbosa, António J. D. Ferreira, and Cristina Galhano. 2022. "Agri-Food Waste as a Method for Weed Control and Soil Amendment in Crops" Agronomy 12, no. 5: 1184. https://doi.org/10.3390/agronomy12051184
APA StyleLorenzo, P., Guilherme, R., Barbosa, S., Ferreira, A. J. D., & Galhano, C. (2022). Agri-Food Waste as a Method for Weed Control and Soil Amendment in Crops. Agronomy, 12(5), 1184. https://doi.org/10.3390/agronomy12051184