Behavior of Vine Varieties Resistant to Fungal Diseases in the Somontano Region
Abstract
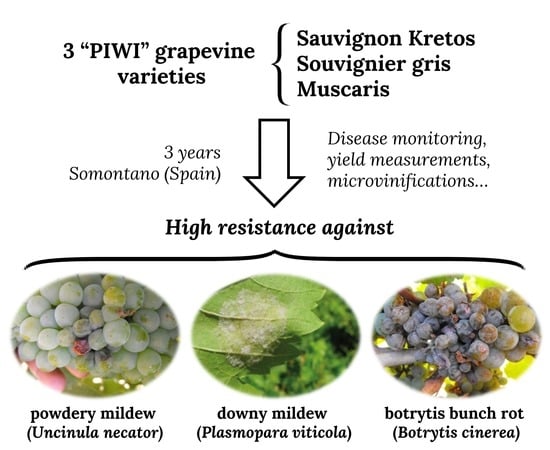
:1. Introduction
2. Material and Methods
2.1. Location
2.2. Plant Material
2.3. Experimental Design and Cultural Techniques
2.4. Data Collection
2.5. Disease Forecast Models
2.6. Winemaking
2.7. Statistical Analyses
3. Results
3.1. Climatic Data
3.2. Phenology and Ampelography
3.3. Disease Monitoring
3.3.1. Powdery Mildew
3.3.2. Downy Mildew
3.3.3. Botrytis Bunch Rot
3.4. Treatment Efficacies and Associated Costs
3.5. Disease Forecast Models
3.6. Production and Ravaz indices
3.7. Initial Values of Musts
3.8. Winemaking Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Galet, P. Précis De Pathologie Viticole, 3rd ed.; Imprimerie JF Impression: Montpellier, France, 1999; p. 296. [Google Scholar]
- Piwi-International. What are Fungus Resistant Grape Varieties (PIWIs)? Available online: https://www.piwi-international.de/en/information-en.html (accessed on 12 September 2019).
- Rousseau, J.; Chanfreau, S.; Bontemps, É. Les Cépages Résistants and Maladies Cryptogamiques; Groupe ICV: Bordeaux, France, 2013; p. 228. [Google Scholar]
- Zito, S.; Caffarra, A.; Richard, Y.; Castel, T.; Bois, B. Climate change and vine protection: The case of mildews management in Burgundy. E3s Web Conf. 2018, 50, 01006. [Google Scholar] [CrossRef]
- Buonassisi, D.; Colombo, M.; Migliaro, D.; Dolzani, C.; Peressotti, E.; Mizzotti, C.; Velasco, R.; Masiero, S.; Perazzolli, M.; Vezzulli, S. Breeding for grapevine downy mildew resistance: A review of “omics” approaches. Euphytica 2017, 213, 103. [Google Scholar] [CrossRef]
- Merdinoglu, D.; Schneider, C.; Prado, E.; Wiedemann-Merdinoglu, S.; Mestre, P. Breeding for durable resistance to downy and powdery mildew in grapevine. OENO One 2018, 52, 203–209. [Google Scholar] [CrossRef] [Green Version]
- VIVC. Table of Loci for Traits in Grapevine Relevant for Breeding. Available online: http://www.vivc.de/docs/dataonbreeding/20180122_Table%20of%20Loci%20for%20Traits%20in%20Grapevine.pdf (accessed on 22 October 2019).
- Sivcev, B.; Sivcev, I.; Rankovic-Vasic, Z. Natural process and use of natural matters in organic viticulture. J. Agric. Sci. Belgrade 2010, 55, 195–215. [Google Scholar] [CrossRef] [Green Version]
- Pomarici, E.; Vecchio, R. Will sustainability shape the future wine market? Wine Econ. Policy 2019, 8, 1–4. [Google Scholar] [CrossRef]
- Pavloušek, P.; Kumšta, M.; Mateiciucová, P. Adaptation of new resistant grapevine varieties to the terroir in the Czech Republic. In X Congrès Internationaux des Terroirs Vitivinicoles; Society of International Terroir Experts in Vitiviniculture: Tokaj-Eger, Hungary, 2014; pp. 1–5. [Google Scholar]
- Siegfried, W.; Temperli, T. Piwi-Reben im Vergleich—ein Zwischenbericht. Schweizerische Zeitschrift für Obst- und Weinbau 2008, 17, 6–9. [Google Scholar]
- Van der Meer, M.; Weibel, F.; Levite, D.; Häseli, A.; Vombach, D. Acceptation des vins de cépages résistants par les consommateurs. Revue suisse de Viticulture, Arboriculture et Horticulture 2010, 42, 147–150. [Google Scholar]
- Becker, A. Piwis in der Praxis: Nicht nur für “Ökos”! Rebe und Wein Weinsberg 2012, 65, 19–22. [Google Scholar]
- Galbrun, C. Etude INRA—Comment réduire ses coûts de Production de 50%. Available online: https://www.reussir.fr/vigne/comment-reduire-ses-couts-de-production-de-50 (accessed on 12 September 2019).
- Fuller, K.B.; Alston, J.M.; Sambucci, O.S. The value of powdery mildew resistance in grapes: Evidence from California. Wine Econ. Policy 2014, 3, 90–107. [Google Scholar] [CrossRef] [Green Version]
- Francesca, S.; Simona, G.; Francesco Nicola, T.; Andrea, R.; Vittorio, R.; Federico, S.; Cynthia, R.; Maria Lodovica, G. Downy mildew (Plasmopara viticola) epidemics on grapevine under climate change. Glob. Chang. Biol. 2006, 12, 1299–1307. [Google Scholar] [CrossRef]
- Sambucci, O.; Alston, J.M.; Fuller, K.B.; Lusk, J. The Pecuniary and nonpecuniary costs of powdery mildew and the potential value of resistant grape varieties in California. Am. J. Enol. Vitic. 2019, 70, 177–187. [Google Scholar] [CrossRef]
- Pennington, T.; Kraus, C.; Alakina, E.; Entling, M.H.; Hoffmann, C. Minimal Pruning and Reduced Plant Protection Promote Predatory Mites in Grapevine. Insects 2017, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Pennington, T.; Reiff, J.M.; Theiss, K.; Entling, M.H.; Hoffmann, C. Reduced fungicide applications improve insect pest control in grapevine. BioControl 2018, 63, 687–695. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Vanden Heuvel, J.E. Influence of grapevine training systems on vine growth and fruit composition: A review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar]
- Barthe, C. Impact de la charge fruitière sur la maturité et la qualité du raisin chez le Seyval blanc et le Vandal-Cliche, deux cépages hybrides cultivés au Québec; Université Laval: Québec, QC, Canada, 2015. [Google Scholar]
- Sun, Q.; Sacks, G.; Lerch, S.; Vanden Heuvel, J.E. Impact of shoot thinning and harvest date on yield components, fruit composition, and wine quality of Marechal Foch. Am. J. Enol. Vitic. 2011, 62, 32–41. [Google Scholar] [CrossRef]
- Pedneault, K.; Provost, C. Fungus resistant grape varieties as a suitable alternative for organic wine production: Benefits, limits, and challenges. Sci. Hortic. 2016, 208, 57–77. [Google Scholar] [CrossRef]
- Yobregat, O. Introduction to resistant vine types: A brief history and overview of the situation. OENO One 2018, 52, 241–246. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Protect. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Lissarrague, J.R.; Baeza, P.; Peiro, E.; Ayuso, J.M.; Cibriain, F.; Blanco, J.A.; Villalba, P. Híbridos resistentes a mildiu-oidio: La apuesta por la sostenibilidad y el respeto medioambiental de VCR. Olint 2016, 29, 6–14. [Google Scholar]
- Badía, D.; Cuchí, J.; Martí, C.; Casanova, J. Los suelos de los viñedos en la D.O. Somontano; Prensas Universitarias de Zaragoza: Zaragoza, Spain, 2006; Volume 8, p. 205. [Google Scholar]
- Morgante, M.; Testolin, R. Nuovi vitigni resistenti alle malattie. In Quaderni Tecnici VCR; Vivai Cooperativi Rauscedo: Rauscedo, Italy, 2019; Volume 15, p. 40. [Google Scholar]
- Vitis International Variety Catalogue VIVC. Souvignier gris Passport Data. Available online: http://www.vivc.de/index.php?r=passport%2Fview&id=22629 (accessed on 12 September 2019).
- Bonnet, P.; Lacombe, T. Le catalogue des vignes cultivées en France. Available online: http://plantgrape.plantnet-project.org/es/nouvelles (accessed on 12 September 2019).
- Vitis International Variety Catalogue VIVC. Muscaris Passport Data. Available online: http://www.vivc.de/index.php?r=passport%2Fview&id=22628 (accessed on 12 September 2019).
- Zini, E.; Dolzani, C.; Stefanini, M.; Gratl, V.; Bettinelli, P.; Nicolini, D.; Betta, G.; Dorigatti, C.; Velasco, R.; Letschka, T.; et al. R-Loci arrangement versus downy and powdery mildew resistance level: A vitis Hybrid survey. Int. J. Mol. Sci. 2019, 20, 3526. [Google Scholar] [CrossRef]
- Vickery, E. Hobby boards moisture meter datalogger. Instruction manual and technical specifications. HobbyBoards: EEUU, 2012. p. 14. Available online: https://web.archive.org/web/20160509003321/http://www.hobby-boards.com/download/manuals/Moisture%20Meter%20Datalogger.pdf (accessed on 22 October 2019).
- Eichhorn, K.W.; Lorenz, D. Phaenologische Entwicklungsstadien der Rebe: Anwendungstermine d. Pflanzenschutzmittel; Sonderdr. aus “Der deutsche Weinbau”; BASF: Ludwigshafen, Germany, 1978. [Google Scholar]
- Baillod, M.; Baggiolini, M. Les stades repères de la vigne. Revue suisse de Viticulture Arboriculture Horticulture 1993, 38, 7–9. [Google Scholar]
- Lorenz, D.; Eichhorn, K.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth stages of the grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- OIV. OIV Descriptor List for Grape Varieties and Vitis Species, 2nd ed.; Organisation Internationale de la Vigne et du Vin: Paris, France, 2009; p. 232. [Google Scholar]
- Townsend, G.R.; Heuberger, J.W. Methods for estimating losses caused by diseases in fungicide experiments. Plant Dis. Report. 1943, 27, 340–343. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 267–268. [Google Scholar] [CrossRef]
- Vasconcelos, M.C.; Castagnoli, S. Leaf canopy structure and vine performance. Am. J. Enol. Vitic. 2000, 51, 390–396. [Google Scholar]
- Goidanich, G. Manuale di Patologia Vegetale; Edizioni Agricole: Bologna, Italy, 1964; Volume 2, p. 1283. [Google Scholar]
- Thomas, C.; Gubler, W.; Leavitt, G. Field testing of a powdery mildew disease forecast model on grapes in California. Phytopathology 1994, 84, 1070. [Google Scholar]
- Nicolini, G.; Roman, T.; Bottura, M.; Gelmetti, A.; Stefanini, M.; Malacarne, M.; Barp, L. In Vides resistentes cultivadas en el Trentino prealpino. Primeros resultados del proyecto VEViR (Resistant vines cultivated in the Trentino pre-Alps. VEViR project first results). In Proceedings of the Sexto congreso internacional sobre la viticultura de montaņa y en fuerte pendiente: Viticultura heroica: De la uva al vino a través de recorridos de sostenibilidad y calidad, La Laguna, Sta. Cruz de Tenerife, Spain, 26–28 April 2018; CERVIM: La Laguna, Sta. Cruz de Tenerife, Spain; pp. 127–134. [Google Scholar]
- Kennelly, M.M.; Gadoury, D.M.; Wilcox, W.F.; Magarey, P.A.; Seem, R.C. Seasonal development of ontogenic resistance to downy mildew in grape berries and rachises. Phytopathology 2005, 95, 1445–1452. [Google Scholar] [CrossRef]
- Gilles, T. Forecasting downy mildew diseases. In Advances in downy Mildew Research—Volume 2; Springer: Dordrecht, The Netherlands, 2004; pp. 35–67. [Google Scholar]
- Lisek, J. Yielding and healthiness of selected grape cultivars for processing in central Poland. J. Fruit Ornam. Plant Res. 2010, 18, 265–272. [Google Scholar]
- Pacifico, D.; Gaiotti, F.; Giusti, M.; Tomasi, D. Performance of interspecific grapevine varieties in north-east Italy. Agric. Sci. 2013, 4, 91–101. [Google Scholar] [Green Version]
- Schwab, A.; Knott, R.; Schottdorf, W. Results from new fungus-tolerant grapevine varieties for Organic Viticulture. In Proceedings the 6th International Congress on Organic Viticulture; Stiftung Ökologie & Landbau: Bad Dürkheim, Germany, 2000; pp. 225–227. [Google Scholar]
- Zanghelini, J.A.; Bogo, A.; Dal Vesco, L.L.; Gomes, B.R.; Mecabo, C.V.; Herpich, C.H.; Welter, L.J. Response of PIWI grapevine cultivars to downy mildew in highland region of southern Brazil. Eur. J. Plant Pathol. 2019, 154, 1051–1058. [Google Scholar] [CrossRef]
- Bonin, B.; de Bem, B.; Brighenti, A.; Wurz, D.; Allebrandt, R.; Brighenti, E.; Araujo, L.; Moretti Ferreira Pinto, F.A.; Bogo, A. Intensity of Anthracnose in resistant varieties (PIWI) in the high altitude regions of southern Brazil. In Proceedings of the 40th World Congress of Vine and Wine, Sofia, Bulgaria, 29 May–2 June 2017; Aurand, J.M., Ed.; 2017; Volume 9. [Google Scholar]
- Zamboni, M.; Bavaresco, L.; Fontana, M.; Vespignani, G. Adaptability of disease resistant grape cultivars to the hilly environment of Emilia Romagna (Italy). Acta Hortic. 2009, 827, 565–570. [Google Scholar] [CrossRef]
- Vezzulli, S.; Vecchione, A.; Stefanini, M.; Zulini, L. Downy mildew resistance evaluation in 28 grapevine hybrids promising for breeding programs in Trentino region (Italy). Eur. J. Plant Pathol. 2018, 150, 485–495. [Google Scholar] [CrossRef]
- Gessler, C.; Pertot, I.; Perazzolli, M. Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar]
- Peressotti, E.; Wiedemann-Merdinoglu, S.; Delmotte, F.; Bellin, D.; Di Gaspero, G.; Testolin, R.; Merdinoglu, D.; Mestre, P. Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety. BMC Plant Biol. 2010, 10, 147. [Google Scholar] [CrossRef]
- AREDVI. Cépages résistants aux maladies cryptogamiques. In Guide des vignobles, Viticulture raisonnée et biologie 2017—Rhône-Mediterranée; IFV., Chambres d’agriculture Languedoc-Roussillon, Provence-Alpes-Côte d’Azur (PACA), Rhône-Alpes, Eds.: Marseille, France, 2017; pp. 99–104. [Google Scholar]
- Savary, S.; Delbac, L.; Rochas, A.; Taisant, G.; Willocquet, L. Analysis of nonlinear relationships in dual epidemics, and its application to the management of grapevine downy and powdery mildews. Phytopathology 2009, 99, 930–942. [Google Scholar] [CrossRef]
- Pedò, S.; Bottura, M.; Porro, D. Development, yield potential and nutritional aspects of resistant grapevine varieties in Trentino Alto Adige. BIO Web Conf. 2019, 13, 02004. [Google Scholar] [CrossRef]
- Smart, R.; Robinson, M. Sunlight into Wine: A Handbook for Winegrape Canopy Management; Winetitles: Adelaide, Australia, 1991; p. 88. [Google Scholar]
- Matthews, M.A. Terroir and other Myths of Winegrowing; University of California Press: Oakland, CA, USA, 2015; p. 308. [Google Scholar]
- Moser, S.; Roman, T.; Versini, L.; Tonidandel, L.; Barchetti, P.; Larcher, R.; Nicolini, G. Distillati sperimentali da vinacce di ibridi resistenti a bacca bianca: Prime valutazioni. L’Enologo 2017, 5, 85–90. [Google Scholar]
- Moser, S.; Roman, T.; Tonidandel, L.; Versini, L.; Nicolini, G.; Larcher, R. Prime osservazioni su grappe sperimentali prodotte in scala micro da uve ibride a bacca bianca. Rivista Internet di Viticoltura ed Enologia 2018, 1/2, 1–7. [Google Scholar]
- Rousseau, J. Les cepages resistants: Présentation, réglementation, disponibilités. Available online: https://www.icv.fr/sites/default/files/ressources/01.cepages_resistants_j_rousseau_icv.pdf (accessed on 12 September 2019).
- Salmon, J.-M.; Ojeda, H.; Escudier, J.-L. Disease resistant grapevine varieties and quality: The case of Bouquet varieties. OENO One 2018, 52, 225–230. [Google Scholar] [CrossRef]
- Espinoza, A.F.; Hubert, A.; Raineau, Y.; Franc, C.; Giraud-Heraud, E. Resistant grape varieties and market acceptance: An evaluation based on experimental economics. OENO One 2018, 52, 247–263. [Google Scholar]
- Schäufele, I.; Hamm, U. Consumers’ perceptions, preferences and willingness-to-pay for wine with sustainability characteristics: A review. J. Clean. Prod. 2017, 147, 379–394. [Google Scholar] [CrossRef]
- Montaigne, E.; Coelho, A.; Khefifi, L. Economic issues and perspectives on innovation in new resistant grapevine varieties in France. Wine Econ. Policy 2016, 5, 73–77. [Google Scholar] [CrossRef]



| Year | March | April | May | June | July | August | September |
| 2016 | 9.3 | 12.5 | 16.1 | 21.6 | 24.9 | 24.0 | 21.4 |
| 2017 | 11.8 | 13.5 | 18.2 | 23.7 | 24.8 | 24.3 | 18.7 |
| 2018 | 8.9 | 13.4 | 17.1 | 22.0 | 25.6 | 25.2 | 22.2 |
| Year | March | April | May | June | July | August | September |
| 2016 | 69.2 | 93.0 | 33.0 | 13.6 | 11.8 | 1.0 | 22.0 |
| 2017 | 109.4 | 34.0 | 45.4 | 47.8 | 23.3 | 7.0 | 27.4 |
| 2018 | 60.7 | 117.0 | 142.6 | 28.4 | 13.0 | 101.9 | 35.4 |
| Variety | 2017 | 2018 | ||||||
|---|---|---|---|---|---|---|---|---|
| 100-Berry Weight (g) | Harvest Weight (kg/vine) | Pruned Wood Weight (kg/vine) | Ravaz Index | 100-Berry Weight (g) | Harvest Weight (kg/vine) | Pruned Wood Weight (kg/vine) | Ravaz Index | |
| Sauvignon Kretos | 95.67 | 3.50 | 0.86 | 4.07 | 99.25 | 3.27 | 0.80 | 4.09 |
| Souvignier gris | 132.50 | 2.03 | 0.15 | 13.53 | 136.30 | 2.27 | 0.49 | 4.63 |
| Muscaris | 132.95 | 1.48 | 0.17 | 8.71 | 128.30 | 2.01 | 0.12 | 16.75 |
| Sauvignon blanc | 130.02 | 2.44 | 0.62 | 3.94 | 151.05 | 3.11 | 0.78 | 3.99 |
| Variety | Year | pH | Total Acidity (g/L) | Probable Alcohol Content (°) | YAN |
|---|---|---|---|---|---|
| Sauvignon Kretos | 2017 | 3.14 ± 0.12 a | 6.61 ± 1.96 | 12.90 ± 1.62 | 223 |
| 2018 | 3.16 ± 0.11 ab | 6.10 ± 1.99 | 13.66 ± 1.80 | 100 | |
| Souvignier gris | 2017 | 3.35 ± 0.09 ab | 6.08 ± 1.00 | 14.30 ± 1.01 | 190 |
| 2018 | 3.38 ± 0.12 ab | 5.50 ± 0.97 | 14.96 ± 1.62 | 84 | |
| Muscaris | 2017 | 3.15 ± 0.11 a | 3.79 ± 1.01 | 16.20 ± 1.12 | 165 |
| 2018 | 3.71 ± 0.10 c | 3.90 ± 1.50 | 15.60 ± 1.33 | 224 | |
| Sauvignon blanc | 2017 | 3.47 ± 0.09 bc | 3.95 ± 1.22 | 13.45 ± 1.10 | 168 |
| 2018 | 3.31 ± 0.07 ab | 4.28 ± 1.35 | 14.01 ± 1.24 | 180 |
| Variety | Year | ABV (%vol) | pH | Total Acidity (g/L) | Volatile Acidity (g/L) | Malic Acid (g/L) | Glucose + Fructose (g/L) |
|---|---|---|---|---|---|---|---|
| Sauvignon Kretos | 2017 | 12.80 | 3.02 | 5.07 | 0.34 | 1.74 | 3.53 |
| 2018 | 14.35 | 3.09 | 6.80 | 0.48 | 1.08 | 1.92 | |
| Sauvignier gris | 2017 | 13.80 | 3.35 | 4.22 | 0.23 | 1.87 | <1 |
| 2018 | 15.55 | 3.41 | 6.01 | 0.80 | 1.95 | 1.41 | |
| Muscaris | 2017 | 18.02 | 3.90 | 2.96 | 0.62 | 1.51 | 2.29 |
| 2018 | 16.22 | 3.54 | 5.72 | 0.58 | 1.55 | 1.39 | |
| Sauvignon blanc | 2017 | 13.05 | 3.39 | 3.52 | 0.27 | 1.65 | 2.45 |
| 2018 | 13.2 | 3.3 | 3.29 | 0.33 | 1.21 | 0.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova-Gascón, J.; Ferrer-Martín, C.; Bernad-Eustaquio, A.; Elbaile-Mur, A.; Ayuso-Rodríguez, J.M.; Torres-Sánchez, S.; Jarne-Casasús, A.; Martín-Ramos, P. Behavior of Vine Varieties Resistant to Fungal Diseases in the Somontano Region. Agronomy 2019, 9, 738. https://doi.org/10.3390/agronomy9110738
Casanova-Gascón J, Ferrer-Martín C, Bernad-Eustaquio A, Elbaile-Mur A, Ayuso-Rodríguez JM, Torres-Sánchez S, Jarne-Casasús A, Martín-Ramos P. Behavior of Vine Varieties Resistant to Fungal Diseases in the Somontano Region. Agronomy. 2019; 9(11):738. https://doi.org/10.3390/agronomy9110738
Chicago/Turabian StyleCasanova-Gascón, José, Carla Ferrer-Martín, Antonio Bernad-Eustaquio, Andrea Elbaile-Mur, José M. Ayuso-Rodríguez, Sergio Torres-Sánchez, Adrián Jarne-Casasús, and Pablo Martín-Ramos. 2019. "Behavior of Vine Varieties Resistant to Fungal Diseases in the Somontano Region" Agronomy 9, no. 11: 738. https://doi.org/10.3390/agronomy9110738
APA StyleCasanova-Gascón, J., Ferrer-Martín, C., Bernad-Eustaquio, A., Elbaile-Mur, A., Ayuso-Rodríguez, J. M., Torres-Sánchez, S., Jarne-Casasús, A., & Martín-Ramos, P. (2019). Behavior of Vine Varieties Resistant to Fungal Diseases in the Somontano Region. Agronomy, 9(11), 738. https://doi.org/10.3390/agronomy9110738