Herpesvirus Antibodies, Vitamin D and Short-Chain Fatty Acids: Their Correlation with Cell Subsets in Multiple Sclerosis Patients and Healthy Controls
Abstract
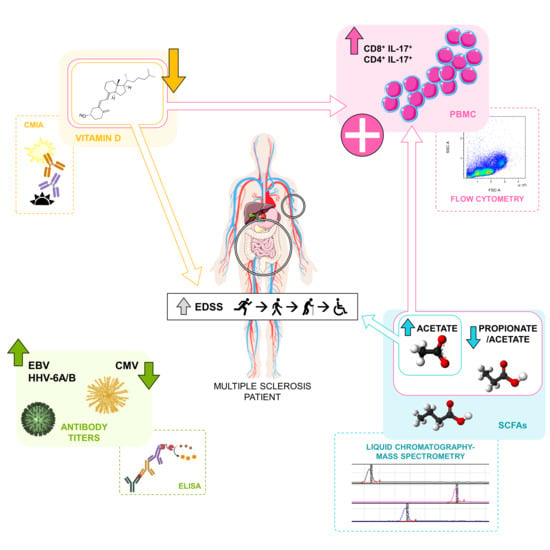
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Collection of Samples
2.3. Liquid Chromatography-Mass Spectrometry (LC-MS/MS) Analysis
2.4. ELISA Analysis
2.5. 25-Hydroxyvitamin D (25(OH)D) Determination
2.6. Monoclonal Antibodies
2.7. In Vitro Stimulation and Labelling of Antigens
2.8. Flow Cytometry
2.9. Clinical Data
2.10. Statistical Analysis
3. Results
3.1. Environmental Factors Included in the Study: Healthy Controls vs. MS Patients
3.2. Correlations among the Environmental Factors Included in the Study
3.2.1. Correlations among the Environmental Factors in Healthy Controls
3.2.2. Correlations among the Environmental Factors in MS Patients
3.3. Correlations among the Environmental Factors Included in the Study with the Demographic and Clinical Data
3.3.1. Correlations among Environmental Factors with Gender and Age in Healthy Controls
3.3.2. Correlations among Environmental Factors with Demographic and Clinical Data in Treated and Untreated MS Patients
3.4. Correlations among the Environmental Factors Included in the Study and the Immune Cells
3.4.1. Correlations with Immune Cells in Healthy Controls
3.4.2. Correlations with Immune Cells in Untreated MS Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geginat, J.; Paroni, M.; Pagani, M.; Galimberti, D.; De Francesco, R.; Scarpini, E.; Abrignani, S. The enigmatic role of viruses in multiple sclerosis: Molecular mimicry or disturbed immune surveillance? Trends Immunol. 2017, 38, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.I.; Munger, K.L.; O’Reilly, E.J.; Falk, K.I.; Ascherio, A. Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann. Neurol. 2010, 67, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Madueño, I.; Garcia-Montojo, M.; Dominguez-Mozo, M.I.; Garcia-Martinez, A.; Arias-Leal, A.M.; Casanova, I.; Arroyo, R.; Alvarez-Lafuente, R. Anti-human herpesvirus 6A/B IgG correlates with relapses and progression in multiple sclerosis. PLoS ONE 2014, 9, e104836. [Google Scholar] [CrossRef] [PubMed]
- Miclea, A.; Bagnoud, M.; Chan, A.; Hoepner, R. A Brief Review of the Effects of Vitamin D on Multiple Sclerosis. Front. Immunol. 2020, 11, 781. [Google Scholar] [CrossRef]
- Arneth, B. Multiple Sclerosis and Smoking. Am. J. Med. 2020, 133, 783–788. [Google Scholar] [CrossRef]
- Kadowaki, A.; Quintana, F.J. The Gut-CNS Axis in Multiple Sclerosis. Trends Neurosci. 2020, 43, 622–634. [Google Scholar] [CrossRef]
- Lünemann, J.D.; Tintoré, M.; Messmer, B.; Strowig, T.; Rovira, A.; Perkal, H.; Caballero, E.; Münz, C.; Montalban, X.; Comabella, M. Elevated Epstein–Barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann. Neurol. 2010, 67, 159–169. [Google Scholar] [CrossRef]
- Alari-Pahissa, E.; Moreira, A.; Zabalza, A.; Álvarez-Lafuente, R.; Munteis, E.; Vera, A.; Arroyo, R.; Álvarez-Cermeño, J.C.; Villar, L.M.; López-Botet, M.; et al. Low cytomegalovirus seroprevalence in early multiple sclerosis: A case for the “hygiene hypothesis”? Eur. J. Neurol. 2018, 25, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.; Alari-Pahissa, E.; Munteis, E.; Vera, A.; Zabalza, A.; Llop, M.; Villarrubia, N.; Costa-García, M.; Álvarez-Lafuente, R.; Villar, L.M.; et al. Adaptive Features of Natural Killer Cells in Multiple Sclerosis. Front. Immunol. 2019, 10, 2403. [Google Scholar] [CrossRef]
- Burton, J.M.; Kimball, S.; Vieth, R.; Bar-Or, A.; Dosch, H.M.; Cheung, R.; Gagne, D.; D’Souza, C.; Ursell, M.; O’Connor, P. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology 2010, 74, 1852–1859. [Google Scholar] [CrossRef] [Green Version]
- Muris, A.H.; Smolders, J.; Rolf, L.; Thewissen, M.; Hupperts, R.; Damoiseaux, J.; SOLARIUM Study Group. Immune regulatory effects of high dose vitamin D supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNβ; the SOLARIUM study. J. NeuroImmunol. 2016, 300, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Junli, G.; Liu, X.; Chen, C.; Sun, X.; Li, H.; Zhou, Y.; Cui, C.; Wang, Y.; Yang, Y.; et al. Gut dysbiosis and lack of short chain fatty acids in a Chinese cohort of patients with multiple sclerosis. Neurochem. Int. 2019, 129, 104468. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Marventano, I.; Barone, M.; La Rosa, F.; Piancone, F.; Mendozzi, L.; d’Arma, A.; Rossi, V.; Pugnetti, L.; Roda, G.; et al. Alterations in Circulating Fatty Acid Are Associated With Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis. Front. Immunol. 2020, 7, 1390. [Google Scholar] [CrossRef]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Eilers, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080.e16. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Update in vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Poser, C.M.; Paty, D.W.; Scheinberg, L.; McDonald, W.I.; Davis, F.A.; Ebers, G.C.; Johnson, K.P.; Sibley, W.A.; Silberberg, D.H.; Tourtellotte, W.W. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann. Neurol. 1983, 13, 227–231. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Noto, D.; Hoshino, Y.; Mizuno, M.; Miyake, S. Butyrate suppresses demyelination and enhances remyelination. J. Neuroinflamm. 2019, 16, 165. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Villa, M.; Sanin, D.E.; Buck, M.D.; O’Sullivan, D.; Ching, R.; Matsushita, M.; Grzes, K.M.; Winkler, F.; Chang, C.-H.; et al. Acetate Promotes T Cell Effector Function during Glucose Restriction. Cell Rep. 2019, 27, 2063–2074.e5. [Google Scholar] [CrossRef] [Green Version]
- Bäcker-Koduah, P.; Bellmann-Strobl, J.; Scheel, M.; Wuerfel, J.; Wernecke, K.D.; Dörr, J.; Brandt, A.U.; Paul, F. Vitamin D and Disease Severity in Multiple Sclerosis-Baseline Data From the Randomized Controlled Trial (EVIDIMS). Front. Neurol. 2020, 11, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, S.R.; Simão, A.N.C.; Alfieri, D.F.; Flauzino, T.; Kallaur, A.P.; Mezzaroba, L.; Lozovoy, M.A.B.; Sabino, B.S.; Ferreira, K.P.Z.; Pereira, W.L.C.J.; et al. Vitamin D deficiency is associated with disability and disease progression in multiple sclerosis patients independently of oxidative and nitrosative stress. J. Neurol. Sci. 2017, 381, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, A.; Boleixa, D.; Reguengo, H.; Samões, R.; Santos, E.; Oliveira, J.C.; Silva, B.; Costa, P.P.; da Silva, A.M. Serum 25-hydroxyvitamin D levels in multiple sclerosis patients from the north of Portugal. J. Steroid Biochem. Mol. Biol. 2018, 180, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, G.; Han, X.; Dong, H.; Geng, J. The association of serum 25-hydroxyvitamin D levels with multiple sclerosis severity and progression in a case-control study from China. J. NeuroImmunol. 2016, 297, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Kępczyńska, K.; Zajda, M.; Lewandowski, Z.; Przedlacki, J.; Zakrzewska-Pniewska, B. Bone metabolism and vitamin D status in patients with multiple sclerosis. Neurol. Neurochir. Pol. 2016, 50, 251–257. [Google Scholar] [CrossRef]
- Thouvenot, E.; Orsini, M.; Daures, J.P.; Camu, W. Vitamin D is associated with degree of disability in patients with fully ambulatory relapsing-remitting multiple sclerosis. Eur. J. Neurol. 2015, 22, 564–569. [Google Scholar] [CrossRef]
- Doosti-Irani, A.; Tamtaji, O.R.; Mansournia, M.A.; Ghayour-Mobarhan, M.; Ferns, G.; Daneshvar Kakhaki, R.; Rezaei Shahmirzadi, A.; Asemi, Z. The effects of vitamin D supplementation on expanded disability status scale in people with multiple sclerosis: A critical, systematic review and metaanalysis of randomized controlled trials. Clin. Neurol. Neurosurg. 2019, 187, 105564. [Google Scholar] [CrossRef]
- Jagannath, V.A.; Filippini, G.; Di Pietrantonj, C.; Asokan, G.V.; Robak, E.W.; Whamond, L.; Robinson, S.A. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst. Rev. 2018, 9, CD008422. [Google Scholar] [CrossRef]
- Chao, Z.; Liang, H.; Lingling, L.; Jie, Z.; Tao, J. The efficacy of vitamin D in multiple sclerosis: A meta-analysis. Mult. Scler. Rel. Dis. 2018, 23, 56–61. [Google Scholar] [CrossRef]
- O’Connell, K.; Sulaimani, J.; Basdeo, S.A.; Kinsella, K.; Jordan, S.; Kenny, O.; Kelly, S.B.; Murphy, D.; Heffernan, E.; Killeen, R.P.; et al. Effects of vitamin D(3) in clinically isolated syndrome and healthy control participants: A double-blind randomised controlled trial. Mult. Scler. J. Exp. Transl. Clin. 2017, 3, 2055217317727296. [Google Scholar] [CrossRef] [PubMed]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-Chain Fatty Acids Regulate Cytokines and Th17/Treg Cells in Human Peripheral Blood Mononuclear Cells in vitro. Immunol. Invest. 2016, 45, 205–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Pérez, S.; Domínguez-Mozo, M.I.; Alonso-Gómez, A.; Medina, S.; Villarrubia, N.; Fernández-Velasco, J.I.; García-Martínez, M.A.; García-Calvo, E.; Estévez, H.; Costa-Frossard, L.; et al. Acetate correlates with disability and immune response in multiple sclerosis. PeerJ 2020, 8, e10220. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S. Recent advances in our understanding of vitamin D regulation of intestinal calcium absorption. Arch. Biochem. Biophys. 2012, 523, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraldi, G.; Fioravanti, A.; De Luca d’Alessandro, E.; Palmery, M.; Martinoli, L. Investigation of the effects of vitamin D and calcium on intestinal motility: In vitro tests and implications for clinical treatment. Acta Pharm. 2015, 65, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordenbo, A.M.; Andersen, J.R.; Andersen, J.T. Disturbances of ano-rectal function in multiple sclerosis. J. Neurol. 1996, 243, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Wiesel, P.H.; Norton, C.; Glickman, S.; Kamm, M.A. Pathophysiology and management of bowel dysfunction in multiple sclerosis. Eur. J. Gastroenterol. Hepatol. 2001, 13, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ghareghani, M.; Reiter, R.J.; Zibara, K.; Farhadi, N. Latitude, Vitamin D, Melatonin, and Gut Microbiota Act in Concert to Initiate Multiple Sclerosis: A New Mechanistic Pathway. Front. Immunol. 2018, 9, 2484. [Google Scholar] [CrossRef]
- Medina, S.; Villarrubia, N.; Sainz de la Maza, S.; Lifante, J.; Costa-Frossard, L.; Roldán, E.; Picón, C.; Álvarez-Cermeño, J.C.; Villar, L.M. Optimal response to dimethyl fumarate associates in MS with a shift from an inflammatory to a tolerogenic blood cell profile. Mult. Scler. J. 2018, 24, 1317–1327. [Google Scholar] [CrossRef]
- Medina, S.; Sainz de la Maza, S.; Villarrubia, N.; Álvarez-Lafuente, R.; Costa-Frossard, L.; Arroyo, R.; Monreal, E.; Tejeda-Velarde, A.; Rodríguez-Martín, E.; Roldán, E.; et al. Teriflunomide induces a tolerogenic bias in blood immune cells of MS patients. Ann. Clin. Transl. Neurol. 2019, 6, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Alenda, R.; Costa-Frossard, L.; Alvarez-Lafuente, R.; Espejo, C.; Rodríguez-Martín, E.; de la Maza, S.S.; Villarrubia, N.; Río, J.; Domínguez-Mozo, M.I.; Montalban, X.; et al. Blood lymphocyte subsets identify optimal responders to IFN-beta in MS. J. Neurol. 2018, 265, 24–31. [Google Scholar] [CrossRef]
- Mellergård, J.; Edström, M.; Jenmalm, M.C.; Dahle, C.; Vrethem, M.; Ernerudh, J. Increased B cell and cytotoxic NK cell proportions and increased T cell responsiveness in blood of natalizumab-treated multiple sclerosis patients. PLoS ONE 2013, 8, e81685. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.; Dandu, N.; Mellergård, J. Treatment effects of fingolimod in multiple sclerosis: Selective changes in peripheral blood lymphocyte subsets. PLoS ONE 2020, 15, e0228380. [Google Scholar] [CrossRef] [PubMed]
- Sellebjerg, F.; Hesse, D.; Limborg, S.; Lund, H.; Søndergaard, H.B.; Krakauer, M.; Sørensen, P.S. Dendritic cell, monocyte and T cell activation and response to glatiramer acetate in multiple sclerosis. Mult. Scler. 2013, 19, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Cunnusamy, K.; Baughman, E.J.; Franco, J.; Ortega, S.B.; Sinha, S.; Chaudhary, P.; Greenberg, B.M.; Frohman, E.M.; Karandikar, N.J. Disease exacerbation of multiple sclerosis is characterized by loss of terminally differentiated autoregulatory CD8+ T cells. Clin. Immunol. 2014, 152, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghadiri, M.; Rezk, A.; Li, R.; Evans, A.; Giacomini, P.S.; Barnett, M.H.; Antel, J.; Bar-Or, A. Pre-treatment T-cell subsets associate with fingolimod treatment responsiveness in multiple sclerosis. Sci Rep. 2020, 10, 356. [Google Scholar] [CrossRef]
- Ratts, R.B.; Lovett-Racke, A.E.; Choy, J.; Northrop, S.C.; Hussain, R.Z.; Karandikar, N.J.; Racke, M.K. CD28−CD57+ T cells predominate in CD8 responses to glatiramer acetate. J. NeuroImmunol. 2006, 178, 117–129. [Google Scholar] [CrossRef]
- Moser, T.; Akgün, K.; Proschmann, U.; Sellner, J.; Ziemssen, T. The role of TH17 cells in multiple sclerosis: Therapeutic implications. Autoimmun. Rev. 2020, 19, 102647. [Google Scholar] [CrossRef]
- Kalra, S.; Lowndes, C.; Durant, L.; Strange, R.C.; Al-Araji, A.; Hawkins, C.P.; Curnow, S.J. Th17 cells increase in RRMS as well as in SPMS, whereas various other phenotypes of Th17 increase in RRMS only. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217319899695. [Google Scholar] [CrossRef] [Green Version]
- Brucklacher-Waldert, V.; Stuerner, K.; Kolster, M.; Wolthausen, J.; Tolosa, E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 2009, 132, 3329–3341. [Google Scholar] [CrossRef]
- Montes, M.; Zhang, X.; Berthelot, L.; Laplaud, D.A.; Brouard, S.; Jin, J.; Rogan, S.; Armao, D.; Jewells, V.; Soulillou, J.P.; et al. Oligoclonal myelin-reactive T-cell infiltrates derived from multiple sclerosis lesions are enriched in Th17 cells. Clin. Immunol. 2009, 130, 133–144. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, D.S.; Hygino, J.; Ferreira, T.B.; Kasahara, T.M.; Barros, P.O.; Monteiro, C.; Oliveira, A.; Tavares, F.; Vasconcelos, C.C.; Alvarenga, R.; et al. Vitamin D modulates different IL-17-secreting T cell subsets in multiple sclerosis patients. J. NeuroImmunol. 2016, 299, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, S.; Gisevius, B.; Duscha, A.; Haghikia, A. Implications of diet and the gut microbiome in neuroinflammatory and neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Huang, Y.; Tian, T.; Wang, P.; Li, Y. Propionate promotes vitamin D receptor expression via yes-associated protein in rats with short bowel syndrome. Biochem. Biophys. Res. Commun. 2020, 523, 645–650. [Google Scholar] [CrossRef]
- Engdahl, E.; Gustafsson, R.; Huang, J.; Biström, M.; Lima Bomfim, I.; Stridh, P.; Khademi, M.; Brenner, N.; Butt, J.; Michel, A.; et al. Increased Serological Response Against Human Herpesvirus 6A Is Associated With Risk for Multiple Sclerosis. Front. Immunol. 2019, 10, 2715. [Google Scholar] [CrossRef] [Green Version]
- Leibovitch, E.C.; Caruso, B.; Ha, S.K.; Schindler, M.K.; Lee, N.J.; Luciano, N.J.; Billioux, B.J.; Guy, J.R.; Yen, C.; Sati, P.; et al. Herpesvirus trigger accelerates neuroinflammation in a nonhuman primate model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2018, 115, 11292–11297. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.; Graham, J.; Akahata, Y.; Oh, U.; Jacobson, S. Mechanism of neuroinflammation: Enhanced cytotoxicity and IL-17 production via CD46 binding. J. Neuroimmune. Pharmacol. 2010, 5, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Mozo, M.I.; Perez-Perez, S.; Villar, L.M.; Oliver-Martos, B.; Villarrubia, N.; Matesanz, F.; Costa-Frossard, L.; Pinto-Medel, M.J.; García-Sánchez, M.I.; Ortega-Madueño, I.; et al. Redictive factors and early biomarkers of response in multiple sclerosis patients treated with natalizumab. Sci. Rep. 2020, 10, 14244. [Google Scholar] [CrossRef]
- Monaghan, K.L.; Wan, E.C.K. The Role of Granulocyte-Macrophage Colony-Stimulating Factor in Murine Models of Multiple Sclerosis. Cells 2020, 9, 611. [Google Scholar] [CrossRef] [Green Version]
- Duncker, P.C.; Stoolman, J.S.; Huber, A.K.; Segal, B.M. GM-CSF Promotes Chronic Disability in Experimental Autoimmune Encephalomyelitis by Altering the Composition of Central Nervous System-Infiltrating Cells, but Is Dispensable for Disease Induction. J. Immunol. 2018, 200, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, R.; Loenenbach, A.; Waterboer, T.; Brenner, N.; Pawlita, M.; Michel, A.; Thamm, M.; Poethko-Müller, C.; Wichmann, O.; Wiese-Posselt, M. Cytomegalovirus (CMV) seroprevalence in the adult population of Germany. PLoS ONE 2018, 13, e0200267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadambari, S.; Klenerman, P.; Pollard, A.J. Why the elderly appear to be more severely affected by COVID-19: The potential role of immunosenescence and CMV. Rev. Med. Virol. 2020, 30, e2144. [Google Scholar] [CrossRef] [PubMed]
- Thewissen, M.; Linsen, L.; Somers, V.; Geusens, P.; Raus, J.; Stinissen, P. Premature immunosenescence in rheumatoid arthritis and multiple sclerosis patients. Ann. N Y Acad. Sci. 2005, 1051, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Langer-Gould, A.; Wu, J.; Lucas, R.; Smith, J.; Gonzales, E.; Amezcua, L.; Haraszti, S.; Hong Chen, L.; Quack, H.; James, J.A.; et al. Epstein-Barr virus, cytomegalovirus, and multiple sclerosis susceptibility: A multiethnic study. Neurology 2017, 89, 1330–1337. [Google Scholar] [CrossRef] [Green Version]
- Makhani, N.; Banwell, B.; Tellier, R.; Yea, C.; McGovern, S.; O’Mahony, J.; Ahorro, J.M.; Arnold, D.; Sadovnick, A.D.; Marrie, R.A.; et al. Viral exposures and MS outcome in a prospective cohort of children with acquired demyelination. Mult. Scler. J. 2016, 22, 385–388. [Google Scholar] [CrossRef]
- Waubant, E.; Mowry, E.M.; Krupp, L.; Chitnis, T.; Yeh, E.A.; Kuntz, N.; Ness, J.; Chabas, D.; Strober, J.; McDonald, J.; et al. Common viruses associated with lower pediatric multiple sclerosis risk. Neurology 2011, 76, 1989–1995. [Google Scholar] [CrossRef] [Green Version]
- Bar-Or, A.; Pender, M.P.; Khanna, R.; Steinman, L.; Hartung, H.P.; Maniar, T.; Croze, E.; Aftab, B.T.; Giovannoni, G.; Joshi, M.A. Epstein-Barr Virus in Multiple Sclerosis: Theory and Emerging Immunotherapies. Trends Mol. Med. 2020, 26, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Marabita, F.; Almgren, M.; Sjöholm, L.K.; Kular, L.; Liu, Y.; James, T.; Kiss, N.B.; Feinberg, A.P.; Olsson, T.; Kockum, I.; et al. Smoking induces DNA methylation changes in Multiple Sclerosis patients with exposure-response relationship. Sci. Rep. 2017, 7, 14589. [Google Scholar] [CrossRef] [Green Version]
- Alrouji, M.; Manouchehrinia, A.; Gran, B.; Constantinescu, C.S. Effects of cigarette smoke on immunity, neuroinflammation and multiple sclerosis. J. NeuroImmunol. 2019, 329, 24–34. [Google Scholar] [CrossRef]





| MS Patients | Controls | |
|---|---|---|
| Females (n (%)) | 129 (67.5%) | 50 (63.3%) |
| Age (years, med (P25–P75)) | 41.0 (35.0–47.5) | 40.0 (34.0–45.5) |
| Age at disease onset (years, med (P25–P75)) | 30.0 (25.0–36.0) | N/A |
| Disease duration at recruitment (months, med (P25–P75)) | 106.0 (56.5–177.5) | N/A |
| MS type: RRMS (n (%)) | 133 (69.6%) | N/A |
| EDSS (med (P25–P75)) | 2.0 (1.0–5.5) | N/A |
| MSSS (med (P25–P75)) | 2.6 (0.9–6.0) | N/A |
| ARR since the beginning of the disease (med (P25–P75))) | 0.6 (0.3–1.1) | N/A |
| Number of relapses two years before (med (P25–P75))) | 1.0 (0.0–2.0) | N/A |
| Non-treated MS patients at recruitment (n (%)) | 57 (29.8%) | N/A |
| Treatment-naïve (n) | 21 | N/A |
| Last treatment for non-naïve (months, med (P25–P75)) | 2.5 (1.0–3.0) | N/A |
| Treated MS patients at recruitment (n (%)) | 134 (70.2%) | N/A |
| MS patients treated with interferon beta | 51 | N/A |
| MS patients treated with glatiramer acetate | 33 | N/A |
| MS patients treated with natalizumab | 33 | N/A |
| MS patients treated with fingolimod | 8 | N/A |
| MS patients treated with teriflunomide | 5 | N/A |
| MS patients treated with dimethyl fumarate | 4 | N/A |
| Treatment duration (med (P25–P75)) | 25.0 (11.0–53.0) | N/A |
| Patients with 9 or more T2 lesions at recruitment (n (%)) * | 135 (91.2%) | N/A |
| Patients with Gd+ lesions at recruitment (n (%)) ** | 26 (20.5%) | N/A |
| HHV-6A/B IgG | HHV-6A/B IgM | EBNA-1 IgG | VCA IgG | CMV IgG | CMV IgM | |
|---|---|---|---|---|---|---|
| MS | 160/182 (87.9%) | 25/179 (14.0%) | 176/186 (94.6%) | 184/185 (99.5%) | 105/171 (61.4%) | 8/135 (5.9%) |
| HC | 52/60 (86.7%) | 5/62 (8.1%) | 54/63 (85.7%) | 60/63 (95.2%) | 37/45 (82.2%) | 0/35 (0%) |
| p | 0.800 | 0.231 | 0.027 | 0.057 | 0.011 | 0.289 |
| OR * (95% CI) ** | 1.12 (0.47–2.66) | 1.85 (0.68–5.07) | 2.93 (1.13–7.59) | 9.2 (0.94–90.12) | 2.91 (1.28–6.63) | 4.73 (0.27–84.02) |
| HHV-6 A/B IgG | HHV-6 A/B IgM | EBNA-1 IgG | VCA IgG | CMV IgG | CMV IgM | 25(OH)D | AA | PA | BA | PA/AA | BA/AA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HHV6A/B IgG 1 | r = 0.176 n.s. | r = 0.123 n.s. | r = 0.180 n.s. | r = 0.145 n.s. | r = 0.087 n.s. | r = −0.008 n.s. | r = −0.058 n.s. | r = −0.024 n.s. | r = −0.202 n.s. | r = −0.017 n.s. | r = −0.119 n.s. | |
| HHV6A/B IgM 1 | r = −0.053 n.s. | r = −0.030 n.s. | r = 0.371 p = 0.026 | r = 0.623 p < 0.0001 | r = 0.265 p = 0.048 | r = 0.028 n.s. | r = −0.046 n.s. | r = −0.080 n.s. | r = −0.028 n.s. | r = −0.148 n.s. | ||
| EBNA1 IgG 1 | r = 0.114 n.s. | r = 0.214 n.s. | r = −0.065 n.s. | r = −0.087 n.s. | r = −0.175 n.s. | r = −0.295 p = 0.044 | r = −0.183 n.s. | r = −0.145 n.s. | r = −0.116 n.s. | |||
| VCA IgG 1 | r = 0.138 n.s. | r = −0.066 n.s. | r = 0.007 n.s. | r = −0.214 n.s. | r = −0.131 n.s. | r = −0.124 n.s. | r = −0.096 n.s. | r = 0.176 n.s. | ||||
| CMV IgG 1 | r = 0.320 n.s. | r = 0.170 n.s. | r = −0.026 n.s. | r = −0.107 n.s. | r = 0.020 n.s. | r = −0.023 n.s. | r = 0.071 n.s. | |||||
| CMV IgM 1 | r = 0.003 n.s. | r = 0.175 n.s. | r = −0.161 n.s. | r = −0.028 n.s. | r = −0.277 n.s. | r = −0.288 n.s. | ||||||
| 25(OH)D 2 | r = −0.142 n.s. | r = 0.242 n.s. | r = 0.203 n.s. | r = 0.370 p = 0.011 | r = 0.406 p = 0.009 | |||||||
| AA 3 | r = 0.450 p = 0.001 | r = 0.364 p = 0.016 | r = 0.364 p = 0.016 | r = −0.482 p = 0.0008 | ||||||||
| PA 3 | r = 0.468 p = 0.003 | r = 0.468 p = 0.003 | r = 0.114 n.s. | |||||||||
| BA 3 | r = −0.016 n.s. | r = 0.570 p < 0.0001 | ||||||||||
| PA/AA | r = −0.549 p = 0.0002 | |||||||||||
| BA/AA |
| HHV-6 A/B IgG | HHV-6 A/B IgM | EBNA-1 IgG | VCA IgG | CMV IgG | CMV IgM | 25(OH)D | AA | PA | BA | PA/AA | BA/AA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HHV6A/BIgG 1 | r = 0.272 p = 0.0002 | r = 0.151 p = 0.039 | r = 0.124 n.s. | r = 0.013 n.s. | r = 0.127 n.s. | r = 0.226 p = 0.023 | r = −0.155 n.s. | r = 0.007 n.s. | r = 0.030 n.s. | r = 0.056 n.s. | r = 0.093 n.s. | |
| HHV6A/BIgM 1 | r = 0.004 n.s. | r = 0.088 n.s. | r = 0.011 n.s. | r = 0.103 n.s. | r = 0.219 p = 0.028 | r = −0.093 n.s. | r = 0.152 n.s. | r = 0.017 n.s. | r = 0.181 n.s. | r = 0.146 n.s. | ||
| EBNA1IgG 1 | r = 0.153 p = 0.036 | r = 0.069 n.s. | r = −0.008 n.s. | r = 0.039 n.s. | r = −0.014 n.s. | r = −0.103 n.s. | r = −0.034 n.s. | r = −0.107 n.s. | r = −0.046 n.s. | |||
| VCAIgG 1 | r = 0.062 n.s. | r = 0.082 n.s. | r = 0.091 n.s. | r = 0.004 n.s. | r = 0.154 n.s. | r = 0.092 n.s. | r = 0.201 n.s. | r = 0.268 p = 0.012 | ||||
| CMVIgG 1 | r = 0.076 n.s. | r = −0.086 n.s. | r = −0.085 n.s. | r = 0.138 n.s. | r = −0.014 n.s. | r = 0.172 n.s. | r = 0.068 n.s. | |||||
| CMVIgM 1 | r = 0.116 n.s. | r = 0.129 n.s. | r = 0.105 n.s. | r = 0.090 n.s. | r = 0.107 n.s. | r = 0.107 n.s. | ||||||
| 25(OH)D 2 | r = −0.216 p = 0.035 | r = 0.167 n.s. | r = 0.129 n.s. | r = 0.365 p = 0.0003 | r = −0.235 p = 0.029 | |||||||
| AA 3 | r = 0.456 p < 0.0001 | r = 0.395 p = 0.0002 | r = −0.386 p = 0.0001 | r = −0.333 p = 0.002 | ||||||||
| PA 3 | r = 0.697 p < 0.0001 | r = 0.527 p < 0.0001 | r = 0.247 p = 0.023 | |||||||||
| BA 3 | r = 0.260 p = 0.017 | r = 0.530 p < 0.0001 | ||||||||||
| PA/AA | r = 0.505 p < 0.0001 | |||||||||||
| BA/AA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez-Mozo, M.I.; Perez-Perez, S.; Villarrubia, N.; Costa-Frossard, L.; Fernandez-Velasco, J.I.; Ortega-Madueño, I.; Garcia-Martinez, M.A.; Garcia-Calvo, E.; Estevez, H.; Luque Garcia, J.L.; et al. Herpesvirus Antibodies, Vitamin D and Short-Chain Fatty Acids: Their Correlation with Cell Subsets in Multiple Sclerosis Patients and Healthy Controls. Cells 2021, 10, 119. https://doi.org/10.3390/cells10010119
Dominguez-Mozo MI, Perez-Perez S, Villarrubia N, Costa-Frossard L, Fernandez-Velasco JI, Ortega-Madueño I, Garcia-Martinez MA, Garcia-Calvo E, Estevez H, Luque Garcia JL, et al. Herpesvirus Antibodies, Vitamin D and Short-Chain Fatty Acids: Their Correlation with Cell Subsets in Multiple Sclerosis Patients and Healthy Controls. Cells. 2021; 10(1):119. https://doi.org/10.3390/cells10010119
Chicago/Turabian StyleDominguez-Mozo, Maria Inmaculada, Silvia Perez-Perez, Noelia Villarrubia, Lucienne Costa-Frossard, Jose Ignacio Fernandez-Velasco, Isabel Ortega-Madueño, Maria Angel Garcia-Martinez, Estefania Garcia-Calvo, Hector Estevez, Jose Luis Luque Garcia, and et al. 2021. "Herpesvirus Antibodies, Vitamin D and Short-Chain Fatty Acids: Their Correlation with Cell Subsets in Multiple Sclerosis Patients and Healthy Controls" Cells 10, no. 1: 119. https://doi.org/10.3390/cells10010119
APA StyleDominguez-Mozo, M. I., Perez-Perez, S., Villarrubia, N., Costa-Frossard, L., Fernandez-Velasco, J. I., Ortega-Madueño, I., Garcia-Martinez, M. A., Garcia-Calvo, E., Estevez, H., Luque Garcia, J. L., Torrejon, M. J., Arroyo, R., Villar, L. M., & Alvarez-Lafuente, R. (2021). Herpesvirus Antibodies, Vitamin D and Short-Chain Fatty Acids: Their Correlation with Cell Subsets in Multiple Sclerosis Patients and Healthy Controls. Cells, 10(1), 119. https://doi.org/10.3390/cells10010119