The Predictive Value of miR-16, -29a and -134 for Early Identification of Gestational Diabetes: A Nested Analysis of the DALI Cohort
Abstract
:1. Introduction
2. Materials and Methods
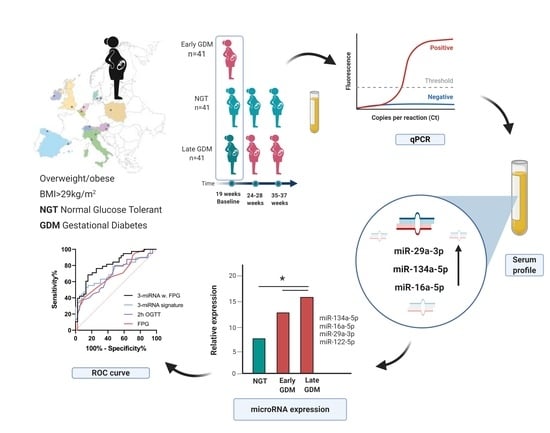
2.1. Participants
2.2. Sample Collection and Storage
2.3. Small Non-Coding RNA Isolation and Analysis
2.4. Ethical Statement
2.5. Statistical Analysis
3. Results
3.1. Anthropometric Baseline Measurement of Study Participants
3.2. Non-Coding RNAs are Associated with GDM
3.3. Correlations between miRNAs and Clinical and Biochemical Phenotypes
3.4. Classification of GDM Cases Using Serum miRNAs
3.5. Predicted Targets and Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Members of the DALI Core Investigator Group
References
- Cheung, N.W.; Byth, K. Population Health Significance of Gestational Diabetes. Diabetes Care 2003, 26, 2005–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Damm, P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: The role of intrauterine hyperglycemia. Diabetes Care 2008, 31, 340–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malcolm, J.C.; Lawson, M.L.; Gaboury, I.; Lough, G.; Keely, E. Glucose tolerance of offspring of mother with gestational diabetes mellitus in a low-risk population. Diabet. Med. 2006, 23, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Boerschmann, H.; Pflüger, M.; Henneberger, L.; Ziegler, A.G.; Hummel, S. Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes Care 2010, 33, 1845–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association Standards of Medical Care in Diabetes—2015 Abridged for Primary Care Providers. Clin. Diabetes 2015, 33, 97–111. [CrossRef] [PubMed] [Green Version]
- Metzger, B.E.; Gabbe, S.; Persson, B. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [Green Version]
- National Institutes of Health. Diagnosing gestational diabetes mellitus. In Proceedings of the NIH Consensus Development Conference Statement, Bethesda, MD, USA, 4–6 March 2013. [Google Scholar]
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; WHO: Geneva, Switzerland; pp. 1–63.
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Filardi, T.; Tavaglione, F.; Di Stasio, M.; Fazio, V.; Lenzi, A.; Morano, S. Impact of risk factors for gestational diabetes (GDM) on pregnancy outcomes in women with GDM. J. Endocrinol. Invest. 2018, 41, 671–676. [Google Scholar] [CrossRef]
- Sovio, U.; Murphy, H.R.; Smith, G.C.S. Accelerated Fetal Growth Prior to Diagnosis of Gestational Diabetes Mellitus: A Prospective Cohort Study of Nulliparous Women. Diabetes Care 2016, 39, 982–987. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, H.D.; Sacks, D.A.; Barbour, L.A.; Feig, D.S.; Catalano, P.M.; Damm, P.; McElduff, A. Issues with the Diagnosis and Classification of Hyperglycemia in Early Pregnancy. Diabetes Care 2016, 39, 53–54. [Google Scholar] [CrossRef] [Green Version]
- Egan, A.M.; Hod, M.; Mahmood, T.; Dunne, F.P. Perspectives on diagnostic strategies for hyperglycaemia in pregnancy—Dealing with the barriers and challenges: Europe. Diabetes Res. Clin. Pr. 2018, 145, 67–72. [Google Scholar] [CrossRef]
- Immanuel, J.; Simmons, D. Screening and Treatment for Early-Onset Gestational Diabetes Mellitus: A Systematic Review and Meta-analysis. Curr. Diab. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Filardi, T.; Catanzaro, G.; Mardente, S.; Zicari, A.; Santangelo, C.; Lenzi, A.; Morano, S.; Ferretti, E. Non-Coding RNA: Role in Gestational Diabetes Pathophysiology and Complications. Int. J. Mol. Sci. 2020, 21, 4020. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef] [Green Version]
- Xiong, D.D.; Lv, J.; Wei, K.L.; Feng, Z.B.; Chen, J.T.; Liu, K.C.; Chen, G.; Luo, D.Z. A nine-miRNA signature as a potential diagnostic marker for breast carcinoma: An integrated study of 1,110 cases. Oncol. Rep. 2017. [Google Scholar] [CrossRef] [Green Version]
- Tüfekci, K.U.; Öner, M.G.; Meuwissen, R.L.J.; Genç, Ş. The role of microRNAs in human diseases. Methods Mol. Biol. 2014. [Google Scholar] [CrossRef]
- Paul, P.; Chakraborty, A.; Sarkar, D.; Langthasa, M.; Rahman, M.; Bari, M.; Singha, R.K.S.; Malakar, A.K.; Chakraborty, S. Interplay between miRNAs and human diseases. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef]
- Liang, Y.; Ridzon, D.; Wong, L.; Chen, C. Characterization of microRNA expression profiles in normal human tissues. Bmc Genom. 2007, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Morales-Prieto, D.M.; Chaiwangyen, W.; Ospina-Prieto, S.; Schneider, U.; Herrmann, J.; Gruhn, B.; Markert, U.R. MicroRNA expression profiles of trophoblastic cells. Placenta 2012, 33, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.G.; Tian, S.; Zhang, L.; Hu, Y.; Guan, C.Y.; Ma, X.; Xia, H.F. The miRNA-29b Is Downregulated in Placenta During Gestational Diabetes Mellitus and May Alter Placenta Development by Regulating Trophoblast Migration and Invasion Through a HIF3A-Dependent Mechanism. Front. Endocrinol. (Lausanne). 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, S.; Li, H.; Wan, J.; Zhou, Q.; Zhou, Y.; Zhang, C. microRNA-96 protects pancreatic β-cell function by targeting PAK1 in gestational diabetes mellitus. BioFactors 2018, 44, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-L.L.; Zhang, L.; Li, J.; Tian, S.; Lv, X.-D.D.; Wang, X.-Q.Q.; Su, X.; Li, Y.; Hu, Y.; Ma, X.; et al. Up-regulation of miR-98 and unraveling regulatory mechanisms in gestational diabetes mellitus. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Strutz, J.; Cvitic, S.; Hackl, H.; Kashofer, K.; Appel, H.M.; Thüringer, A.; Desoye, G.; Koolwijk, P.; Hiden, U. Gestational diabetes alters microRNA signatures in human feto-placental endothelial cells depending on fetal sex. Clin. Sci. 2018, 132, 2437–2449. [Google Scholar] [CrossRef]
- Gillet, V.; Ouellet, A.; Stepanov, Y.; Rodosthenous, R.S.; Croft, E.K.; Brennan, K.; Abdelouahab, N.; Baccarelli, A.; Takser, L. miRNA Profiles in Extracellular Vesicles From Serum Early in Pregnancies Complicated by Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2019, 104, 5154–5166. [Google Scholar] [CrossRef]
- Ma, T.; Jiang, H.; Gao, Y.; Zhao, Y.; Dai, L.; Xiong, Q.; Xu, Y.; Zhao, Z.; Zhang, J. Microarray analysis of differentially expressed microRNAs in non-regressed and regressed bovine corpus luteum tissue; microRNA-378 may suppress luteal cell apoptosis by targeting the interferon gamma receptor 1 gene. J. Appl. Genet. 2011, 52, 481–486. [Google Scholar] [CrossRef]
- Martino, F.; Magenta, A.; Pannarale, G.; Martino, E.; Zanoni, C.; Perla, F.M.; Puddu, P.E.; Barillà, F.; Mughal, W.; Nguyen, L.; et al. MEG3 damages fetal endothelial function induced by gestational diabetes mellitus via AKT pathway. Front. Endocrinol. (Lausanne). 2019, 9, 156–159. [Google Scholar] [CrossRef]
- Hocaoglu, M.; Demirer, S.; Loclar Karaalp, I.; Kaynak, E.; Attar, E.; Turgut, A.; Karateke, A.; Komurcu-Bayrak, E. Identification of miR-16-5p and miR-155-5p microRNAs differentially expressed in circulating leukocytes of pregnant women with polycystic ovary syndrome and gestational diabetes. Gynecol. Endocrinol. 2020, 1–5. [Google Scholar] [CrossRef]
- Hocaoglu, M.; Demirer, S.; Senturk, H.; Turgut, A.; Komurcu-Bayrak, E. Differential expression of candidate circulating microRNAs in maternal blood leukocytes of the patients with preeclampsia and gestational diabetes mellitus. Pregnancy Hypertens. 2019, 17, 5–11. [Google Scholar] [CrossRef]
- Zhao, C.; Dong, J.; Jiang, T.; Shi, Z.; Yu, B.; Zhu, Y.; Chen, D.; Xu, J.; Huo, R.; Dai, J.; et al. Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. PLoS ONE 2011, 6, e23925. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ibarra, A.; Martínez-Razo, L.D.; Vázquez-Martínez, E.R.; Martínez-Cruz, N.; Flores-Ramírez, R.; García-Gómez, E.; López-López, M.; Ortega-González, C.; Camacho-Arroyo, I.; Cerbón, M. Unhealthy levels of phthalates and bisphenol a in mexican pregnant women with gestational diabetes and its association to altered expression of miRNAs involved with metabolic disease. Int. J. Mol. Sci. 2019, 20, 3343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoffe, L.; Polsky, A.; Gilam, A.; Raff, C.; Mecacci, F.; Ognibene, A.; Crispi, F.; Gratacós, E.; Kanety, H.; Mazaki-Tovi, S.; et al. Early diagnosis of gestational diabetes mellitus using circulating microRNAs. Eur. J. Endocrinol. 2019, 181, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Badosa, G.; Bonmatí, A.; Ortega, F.-J.; Mercader, J.-M.; Guindo-Martínez, M.; Torrents, D.; Prats-Puig, A.; Martinez-Calcerrada, J.-M.; Platero-Gutierrez, E.; De Zegher, F.; et al. Altered Circulating miRNA Expression Profile in Pregestational and Gestational Obesity. J. Clin. Endocrinol. Metab. 2015, 100, E1446–E1456. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Lv, J.; Xu, P.; Gu, L.; Cao, J.; Xu, L.; Xue, K.; Li, Q. Identification of microRNAs and genes associated with hyperandrogenism in the follicular fluid of women with polycystic ovary syndrome. J. Cell. Biochem. 2017, 3913–3921. [Google Scholar] [CrossRef]
- Wander, P.L.; Boyko, E.J.; Hevner, K.; Parikh, V.J.; Tadesse, M.G.; Sorensen, T.K.; Williams, M.A.; Enquobahrie, D.A. Circulating early- and mid-pregnancy microRNAs and risk of gestational diabetes. Diabetes Res. Clin. Pr. 2017, 132, 1–9. [Google Scholar] [CrossRef]
- Sebastiani, G.; Guarino, E.; Grieco, G.E.; Formichi, C.; Poggi, C.D.; Ceccarelli, E.; Dotta, F. Circulating microRNA (miRNA) expression profiling in plasma of patients with gestational diabetes mellitus reveals upregulation of miRNA miR-330-3p. Front. Endocrinol. (Lausanne). 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lamadrid-Romero, M.; Solís, K.H.; Cruz-Reséndiz, M.S.; Pérez, J.E.; Díaz, N.F.; Flores-Herrera, H.; García-López, G.; Perichart, O.; Reyes-Muñoz, E.; Arenas-Huertero, F.; et al. Central nervous system development-related microRNAs levels increase in the serum of gestational diabetic women during the first trimester of pregnancy. Neurosci. Res. 2018, 130, 8–22. [Google Scholar] [CrossRef]
- Nair, S.; Jayabalan, N.; Guanzon, D.; Palma, C.; Scholz-Romero, K.; Elfeky, O.; Zuñiga, F.; Ormazabal, V.; Diaz, E.; Rice, G.E.; et al. Human placental exosomes in gestational diabetes mellitus carry a specific set of miRNAs associated with skeletal muscle insulin sensitivity. Clin. Sci. 2018, 132, 2451–2467. [Google Scholar] [CrossRef]
- Pheiffer, C.; Dias, S.; Rheeder, P.; Adam, S. Decreased Expression of Circulating miR-20a-5p in South African Women with Gestational Diabetes Mellitus. Mol. Diagn. 2018, 22, 345–352. [Google Scholar] [CrossRef]
- Tagoma, A.; Alnek, K.; Kirss, A.; Uibo, R.; Haller-Kikkatalo, K. MicroRNA profiling of second trimester maternal plasma shows upregulation of miR-195-5p in patients with gestational diabetes. Gene 2018, 672, 137–142. [Google Scholar] [CrossRef]
- Jelsma, J.G.M.; van Poppel, M.N.M.; Galjaard, S.; Desoye, G.; Corcoy, R.; Devlieger, R.; van Assche, A.; Timmerman, D.; Jans, G.; Harreiter, J.; et al. DALI: Vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention: An European multicentre, randomised trial—study protocol. Bmc Pregnancy Childbirth 2013, 13, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, D.; Devlieger, R.; van Assche, A.; Jans, G.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Desoye, G.; Harreiter, J.; et al. Effect of Physical Activity and/or Healthy Eating on GDM Risk: The DALI Lifestyle Study. J. Clin. Endocrinol. Metab. 2017, 102, 903–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoy, R.; Mendoza, L.C.; Simmons, D.; Desoye, G.; Adelantado, J.M.; Chico, A.; Devlieger, R.; van Assche, A.; Galjaard, S.; Timmerman, D.; et al. The DALI vitamin D randomized controlled trial for gestational diabetes mellitus prevention: No major benefit shown besides vitamin D sufficiency. Clin. Nutr. 2020, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000. [Google Scholar] [CrossRef]
- Mari, A.; Pacini, G.; Murphy, E.; Ludvik, B.; Nolan, J.J. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999. [Google Scholar] [CrossRef]
- Patarrão, R.S.; Wayne Lautt, W.; Paula Macedo, M. Assessment of methods and indexes of insulin sensitivity. Rev. Port. Endocrinol. Diabetes E Metab. 2014. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, 1–38. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Zhu, Y.; Tian, F.; Li, H.; Zhou, Y.; Lu, J.; Ge, Q. Profiling maternal plasma microRNA expression in early pregnancy to predict gestational diabetes mellitus. Int. J. Gynecol. Obs. 2015, 130, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Zhu, Y.; Li, H.; Tian, F.; Xie, X.; Bai, Y. Differential expression of circulating miRNAs in maternal plasma in pregnancies with fetal macrosomia. Int. J. Mol. Med. 2015, 35, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.L.; Jia, Y.J.; Xing, B.H.; Shi, D.D.; Dong, X.J. Plasma microRNA-16-5p, -17-5p and -20a-5p: Novel diagnostic biomarkers for gestational diabetes mellitus. J. Obs. Gynaecol. Res. 2017, 43, 974–981. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Kroh, E.; Wood, B.; Arroyo, D.J.; Dougherty, J.K.; Miyaji, M.M.; Tait, F.J.; Tewari, M. Blood Cell Origin of Circulating MicroRNAs: A Cautionary Note for Cancer Biomarkers Studies. Cancer Prev. Res. 2012, 5, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.Z.; Dong, J.; Zhang, J.; Wang, S.; He, Y.; Yan, Y.X. Identification of neuroendocrine stress response-related circulating MicroRNAs as biomarkers for type 2 diabetes mellitus and insulin resistance. Front. Endocrinol. (Lausanne). 2018. [Google Scholar] [CrossRef]
- Khaliq, O.P.; Murugesan, S.; Moodley, J.; Mackraj, I. Differential expression of miRNAs are associated with the insulin signaling pathway in preeclampsia and gestational hypertension. Clin. Exp. Hypertens. 2018. [Google Scholar] [CrossRef]
- Salomon, C.; Scholz-Romero, K.; Sarker, S.; Sweeney, E.; Kobayashi, M.; Correa, P.; Longo, S.; Duncombe, G.; Mitchell, M.D.; Rice, G.E.; et al. Gestational Diabetes Mellitus Is Associated With Changes in the Concentration and Bioactivity of Placenta-Derived Exosomes in Maternal Circulation Across Gestation. Diabetes 2016, 65, 598–609. [Google Scholar] [CrossRef] [Green Version]
- Mattis, A.N.; Song, G.; Hitchner, K.; Kim, R.Y.; Lee, A.Y.; Sharma, A.D.; Malato, Y.; Mcmanus, M.T.; Esau, C.C.; Koller, E.; et al. A screen in mice uncovers repression of lipoprotein lipase by microRNA-29a as a mechanism for lipid distribution away from the liver. Hepatology 2015. [Google Scholar] [CrossRef] [Green Version]
- Dooley, J.; Garcia-Perez, J.E.; Sreenivasan, J.; Schlenner, S.M.; Vangoitsenhoven, R.; Papadopoulou, A.S.; Tian, L.; Schonefeldt, S.; Serneels, L.; Deroose, C.; et al. The microRNA-29 family dictates the balance between homeostatic and pathological glucose handling in diabetes and obesity. Diabetes 2016. [Google Scholar] [CrossRef] [Green Version]
- Bagge, A.; Clausen, T.R.; Larsen, S.; Ladefoged, M.; Rosenstierne, M.W.; Larsen, L.; Vang, O.; Nielsen, J.H.; Dalgaard, L.T. MicroRNA-29a is up-regulated in beta-cells by glucose and decreases glucose-stimulated insulin secretion. Biochem. Biophys. Res. Commun. 2012, 426, 266–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, B.M.; Lockstone, H.E.; Taylor, J.M.; Ria, M.; Barrett, A.; Collins, S.; Kaisaki, P.; Argoud, K.; Fernandez, C.; Travers, M.E.; et al. Global microRNA expression profiles in insulin target tissues in a spontaneous rat model of type 2 diabetes. Diabetologia 2010, 53, 1099–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udesen, P.B.; Glintborg, D.; Sørensen, A.E.; Svendsen, R.; Nielsen, N.L.S.; Wissing, M.L.M.; Andersen, M.S.; Englund, A.L.M.; Dalgaard, L.T. Metformin decreases miR-122, miR-223 and miR-29a in women with polycystic ovary syndrome. Endocr. Connect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rees, W.D.; Wilson, F.A.; Maloney, C.A. Sulfur amino acid metabolism in pregnancy: The impact of methionine in the maternal diet. J. Nutr. 2006, 136, 1701S–1705S. [Google Scholar]
- Lassance, L.; Miedl, H.; Absenger, M.; Diaz-Perez, F.; Lang, U.; Desoye, G.; Hiden, U. Hyperinsulinemia Stimulates Angiogenesis of Human Fetoplacental Endothelial Cells: A Possible Role of Insulin in Placental Hypervascularization in Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2013, 98, E1438–E1447. [Google Scholar] [CrossRef] [Green Version]
- Hiden, U.; Lassance, L.; Tabrizi, N.G.; Miedl, H.; Tam-Amersdorfer, C.; Cetin, I.; Lang, U.; Desoye, G. Fetal Insulin and IGF-II Contribute to Gestational Diabetes Mellitus (GDM)-Associated Up-Regulation of Membrane-Type Matrix Metalloproteinase 1 (MT1-MMP) in the Human Feto-Placental Endothelium. J. Clin. Endocrinol. Metab. 2012, 97, 3613–3621. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Wu, Z.; Dai, Z.; Sun, K.; Wang, J.; Wu, G. Nutritional epigenetics with a focus on amino acids: Implications for the development and treatment of metabolic syndrome. J. Nutr. Biochem. 2016. [Google Scholar] [CrossRef]
- Freyberg, Z.; Saavedra, J.M. Trace Amines and Trace Amine-Associated Receptors: A New Frontier in Cell Signaling. Cell. Mol. Neurobiol. 2020. [Google Scholar] [CrossRef]
- Williams, Z.; Ben-Dov, I.Z.; Elias, R.; Mihailovic, A.; Brown, M.; Rosenwaks, Z.; Tuschl, T. Comprehensive profiling of circulating microRNA via small RNA sequencing of cDNA libraries reveals biomarker potential and limitations. Proc. Natl. Acad. Sci. 2013, 110, 4255–4260. [Google Scholar] [CrossRef] [Green Version]
- Schratt, G.M.; Tuebing, F.; Nigh, E.A.; Kane, C.G.; Sabatini, M.E.; Kiebler, M.; Greenberg, M.E. A brain-specific microRNA regulates dendritic spine development. Nature 2006, 439, 283–289. [Google Scholar] [CrossRef]
- Wang, H.W.; Su, S.H.; Wang, Y.L.; Chang, S.T.; Liao, K.H.; Lo, H.H.; Chiu, Y.L.; Hsieh, T.H.; Huang, T.S.; Lin, C.S.; et al. MicroRNA-134 contributes to glucose-induced endothelial cell dysfunction and this effect can be reversed by far-infrared irradiation. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Jaskolka, D.; Retnakaran, R.; Zinman, B.; Kramer, C.K. Sex of the baby and risk of gestational diabetes mellitus in the mother: A systematic review and meta-analysis. Diabetologia 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, S.; Cao, N.; Tang, Y.; Gu, W. Identification of key microRNAs and genes in preeclampsia by bioinformatics analysis. PLoS ONE 2017. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.X.; Chen, B.; Li, Q.X.; Liang, Y.C. MiR-134 inhibits infiltration of trophoblast cells in placenta of patients with preeclampsia by decreasing ITGB1 expression. Eur. Rev. Med. Pharm. Sci. 2018. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Hauguel-De Mouzon, S.; Lepercq, J.; Challier, J.-C.; Huston-Presley, L.; Friedman, J.E.; Kalhan, S.C.; Catalano, P.M. TNF- Is a Predictor of Insulin Resistance in Human Pregnancy. Diabetes 2002, 51, 2207–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, G.; Xie, W.; Li, L.; Zhang, M.; Liu, D.; Tan, Y.L.; Cheng, H.P.; Gong, D.; Huang, C.; Zheng, X.L.; et al. MicroRNA-134 actives lipoprotein lipase-mediated lipid accumulation and inflammatory response by targeting angiopoietin-like 4 in THP-1 macrophages. Biochem. Biophys. Res. Commun. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ameling, S.; Kacprowski, T.; Chilukoti, R.K.; Malsch, C.; Liebscher, V.; Suhre, K.; Pietzner, M.; Friedrich, N.; Homuth, G.; Hammer, E.; et al. Associations of circulating plasma microRNAs with age, body mass index and sex in a population-based study. Bmc Med. Genom. 2015. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Hong, J.; Cao, Y.; Shi, J.; Gu, W.; Ning, G.; Zhang, Y.; Wang, W. Elevated circulating microRNA-122 is associated with obesity and insulin resistance in young adults. Eur. J. Endocrinol. 2015. [Google Scholar] [CrossRef]
- Willeit, P.; Skroblin, P.; Moschen, A.R.; Yin, X.; Kaudewitz, D.; Zampetaki, A.; Barwari, T.; Whitehead, M.; Ramírez, C.M.; Goedeke, L.; et al. Circulating MicroRNA-122 Is Associated With the Risk of New-Onset Metabolic Syndrome and Type 2 Diabetes. Diabetes 2017, 66, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Hess, A.L.; Larsen, L.H.; Udesen, P.B.; Sanz, Y.; Larsen, T.M.; Dalgaard, L.T. Levels of Circulating miR-122 are Associated with Weight Loss and Metabolic Syndrome. Obesity 2020. [Google Scholar] [CrossRef]
- Fornes, D.; Heinecke, F.; Roberti, S.L.; White, V.; Capobianco, E.; Jawerbaum, A. Proinflammation in maternal and fetal livers and circulating miR-122 dysregulation in a GDM rat model induced by intrauterine programming. Mol. Cell. Endocrinol. 2020, 510, 110824. [Google Scholar] [CrossRef]
- Harreiter, J.; Desoye, G.; van Poppel, M.N.M.; Kautzky-Willer, A.; Dunne, F.; Corcoy, R.; Devlieger, R.; Simmons, D.; Adelantado, J.M.; Damm, P.; et al. The Effects of Lifestyle and/or Vitamin D Supplementation Interventions on Pregnancy Outcomes: What Have We Learned from the DALI Studies? Curr. Diab. Rep. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Harreiter, J.; Simmons, D.; Desoye, G.; Corcoy, R.; Adelantado, J.M.; Devlieger, R.; van Assche, A.; Galjaard, S.; Damm, P.; Mathiesen, E.R.; et al. IADPSG and WHO 2013 Gestational Diabetes Mellitus Criteria Identify Obese Women With Marked Insulin Resistance in Early Pregnancy. Diabetes Care 2016, 39, e90–e92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huvinen, E.; Eriksson, J.G.; Stach-Lempinen, B.; Tiitinen, A.; Koivusalo, S.B. Heterogeneity of gestational diabetes (GDM) and challenges in developing a GDM risk score. Acta Diabetol. 2018, 55, 1251–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powe, C.E.; Hivert, M.-F.; Udler, M.S. Defining Heterogeneity Among Women With Gestational Diabetes Mellitus. Diabetes 2020, 69, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Luque-Fernandez, M.A.; Bogdanet, D.; Desoye, G.; Dunne, F.; Halperin, J.A. Plasma Glycated CD59 Predicts Early Gestational Diabetes and Large for Gestational Age Newborns. J. Clin. Endocrinol. Metab. 2020, 105, e1033–e1040. [Google Scholar] [CrossRef]
- Immanuel, J.; Simmons, D.; Desoye, G.; Corcoy, R.; Adelantado, J.M.; Devlieger, R.; Lapolla, A.; Dalfra, M.G.; Bertolotto, A.; Harreiter, J.; et al. Performance of early pregnancy HbA1c for predicting gestational diabetes mellitus and adverse pregnancy outcomes in obese European women. Diabetes Res. Clin. Pr. 2020, 168, 108378. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.C.; Harreiter, J.; Simmons, D.; Desoye, G.; Adelantado, J.M.; Juarez, F.; Chico, A.; Devlieger, R.; Van Assche, A.; Galjaard, S.; et al. Risk factors for hyperglycemia in pregnancy in the DALI study differ by period of pregnancy and OGTT time point. Eur. J. Endocrinol. 2018, 179, 39–49. [Google Scholar] [CrossRef]
- van Hoorn, F.; Koster, M.P.H.; Naaktgeboren, C.A.; Groenendaal, F.; Kwee, A.; Lamain-de Ruiter, M.; Franx, A.; Bekker, M.N. Prognostic models versus single risk factor approach in first-trimester selective screening for gestational diabetes mellitus: A prospective population-based multicentre cohort study. Bjog An. Int. J. Obs. Gynaecol. 2020, 1–10. [Google Scholar] [CrossRef]




| NGT (n = 41) | Early GDM (n = 41) | Late GDM (n = 41) | NGT vs. Early GDM | NGT vs. Late GDM | Early GDM vs. Late GDM | |
|---|---|---|---|---|---|---|
| Gestational age at inclusion (weeks) | 15.2 ± 2.4 | 14.9 ± 2.4 | 15.3 ± 2.5 | 1.0 | 1.0 | 1.0 |
| Gestational age at delivery (weeks) | 40.1 ± 1.1 | 39.3 ± 1.7 | 40 ± 39.3 | 0.17 | 0.67 | 0.92 |
| Age (years) | 33.2 ± 3.8 | 33.7 ± 4 | 32.7 ± 4 | 1.0 | 1.0 | 0.73 |
| BMI at inclusion (kg/m2) | 33.3 (32.2–35.4) | 33.3 (31.7−36.0) | 33.3 (31.7−35.9) | 0.99 | 0.99 | 0.99 |
| Waist circumference (cm) | 107 ± 10.7 | 107.7 ± 9.8 | 105.6 ± 8.8 | 1.0 | 1.0 | 1.0 |
| Neck circumference (cm) | 36.0 (35.2–37.0) | 36.0 (34.5−38.0) | 35.5 (34.6−36.9) | 0.58 | 0.58 | 0.58 |
| Resting heart rate (RHR) | 79.7 ± 11.3 | 83.9 ± 12.2 | 80.0 ± 9.3 | 0.28 | 1.0 | 0.32 |
| Fasting glucose (mmol/L) | 4.7 (4.3–4.8) | 5.2 (5−5.6) | 4.9 (4.6−5.2) | <0.001 | 0.024 | 0.002 |
| 1h glucose (mmol/L) | 7.2 (5.9–7.6) | 9.0 (7.2−10.3) | 7.2 (6.2−8.3) | <0.001 | 1.0 | 0.002 |
| 2h glucose (mmol/L) | 5.6 (4.9–6.5) | 7.3 (6.2 − 8.4) | 6.4 (5.7−7.4) | <0.001 | 0.024 | 0.14 |
| HbA1c (%) | 5.0 (4.8–5.4) | 5.1(5−5.4) | 5.2 (5.0−5.5) | 0.16 | 0.16 | 0.16 |
| Fasting insulin (μU/mL) | 11.9 (9.7–18.4) | 18.1 (13.9−25.8) | 14.6 (9.4−18.5) | 0.004 | 1.0 | 0.027 |
| 1h insulin (μU/mL) | 95.2 (57.5–161.1) | 127.7 (66.2−173) | 95.8 (52.5−175.5) | 0.45 | 0.45 | 0.45 |
| 2h insulin (μU/mL) | 52.2 (34.5–88.1) | 92.4 (58.4−153.7) | 59.8 (46.9−100.7) | 0.001 | 0.59 | 0.078 |
| HOMA-IR | 2.61 (1.98−3.70) | 4.25 (3.19−6.53) | 3.11 (2.13−4.19) | <0.001 | 0.87 | 0.006 |
| Quicki | 0.330 ± 0.02 | 0.309 ± 0.021 | 0.325 ± 0.027 | <0.001 | 0.94 | 0.005 |
| OGIS (ml/min/m2) | 424.9 ± 56.9 | 328.7 ± 67.7 | 385.1 ± 52.8 | <0.001 | 0.10 | 0.022 |
| Matsuda | 2.98 (2.3−4.4) | 2.1 (1.0−3.0) | 2.83 (2.1−3.9) | 0.061 | 1.0 | 0.61 |
| Triglycerides (mmol/L) | 1.32 (1.13−1.60) | 1.48 (1.07−2.04) | 1.27 (0.9−1.68) | 0.32 | 0.32 | 0.32 |
| Free fatty acids (mmol/L) | 0.61 ± 0.19 | 0.62 ± 0.22 | 0.65 ± 0.17 | 1.0 | 1.0 | 1.0 |
| HDL cholesterol (mmol/L) | 1.46 ± 0.23 | 1.4 ± 0.27 | 1.49 ± 0.26 | 0.93 | 1.0 | 0.34 |
| LDL cholesterol (mmol/L) | 3.16 ± 0.81 | 2.98 ± 0.77 | 3.32 ± 0.75 | 0.94 | 1.0 | 0.17 |
| Leptin (pg/mL) | 36.0 (25.2−46.1) | 31.9 (23.1−43.6) | 38.9 (29.5−47.3) | 0.53 | 0.53 | 0.53 |
| Ethnicity (European/non-European) | 37/4 | 33/8 | 32/9 | 0.35 | 0.23 | 1.0 |
| Parity (Primipara/Multipara) | 18/23 | 24/17 | 26/15 | 0.27 | 0.12 | 0.82 |
| Previous GDM (No/Yes) | 32/1 | 22/5 | 21/0 | 0.081 | 1.0 | 0.059 |
| Smoking (No/Yes) | 37/4 | 36/5 | 36/5 | 1.0 | 1.0 | 0.92 |
| Offspring sex (Male/Female) | 23/18 | 11/15 | 20/21 | 0.54 | 0.66 | 0.63 |
| Birthweight (g) * | 3548 ± 478 | 3441 ± 598 | 3557 ± 496 | 1.0 | 1.0 | 1.0 |
| miR-29a-3p | miR-134-5p | miR-16-5p | miR-122-5p | |
|---|---|---|---|---|
| Age a | 0.133 | 0.114 | 0.203 * | 0.077 |
| 1h glucose a | 0.191 * | 0.101 | 0.256 *,b | 0.136 |
| 2h glucose b | 0.266 * | 0.226* | 0.345 ** | 0.069 |
| HbA1c b | 0.211 | 0.044 | 0.259 * | 0.016 |
| Matsuda index b | 0.125 | 0.131 | 0.082 | −0.309 * |
| Insulin sensitivity (OGIS) b | −0.040 | 0.016 | −0.054 | −0.437 ** |
| Triglycerides a | −0.088 | −0.106 | −0.197 * | 0.030 |
| Free fatty acids a | 0.186 * | 0.059 | −0.006 | 0.034 |
| HDL cholesterol c | −0.085 | −0.091 | −0.106 | −0.240 * |
| Leptin c | 0.102 | 0.132 | 0.058 | −0.236 * |
| Birthweight | 0.061 | 0.068 | 0.047 | 0.243 * |
| miR-134-5p a | 0.731 *** | - | - | - |
| miR-16-5p a | 0.487 *** | 0.181 | - | - |
| miR-122-5p a | 0.173 | 0.050 | 0.343 *** | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sørensen, A.E.; van Poppel, M.N.M.; Desoye, G.; Damm, P.; Simmons, D.; Jensen, D.M.; Dalgaard, L.T.; The DALI Core Investigator Group. The Predictive Value of miR-16, -29a and -134 for Early Identification of Gestational Diabetes: A Nested Analysis of the DALI Cohort. Cells 2021, 10, 170. https://doi.org/10.3390/cells10010170
Sørensen AE, van Poppel MNM, Desoye G, Damm P, Simmons D, Jensen DM, Dalgaard LT, The DALI Core Investigator Group. The Predictive Value of miR-16, -29a and -134 for Early Identification of Gestational Diabetes: A Nested Analysis of the DALI Cohort. Cells. 2021; 10(1):170. https://doi.org/10.3390/cells10010170
Chicago/Turabian StyleSørensen, Anja Elaine, Mireille N.M. van Poppel, Gernot Desoye, Peter Damm, David Simmons, Dorte Møller Jensen, Louise Torp Dalgaard, and The DALI Core Investigator Group. 2021. "The Predictive Value of miR-16, -29a and -134 for Early Identification of Gestational Diabetes: A Nested Analysis of the DALI Cohort" Cells 10, no. 1: 170. https://doi.org/10.3390/cells10010170
APA StyleSørensen, A. E., van Poppel, M. N. M., Desoye, G., Damm, P., Simmons, D., Jensen, D. M., Dalgaard, L. T., & The DALI Core Investigator Group. (2021). The Predictive Value of miR-16, -29a and -134 for Early Identification of Gestational Diabetes: A Nested Analysis of the DALI Cohort. Cells, 10(1), 170. https://doi.org/10.3390/cells10010170