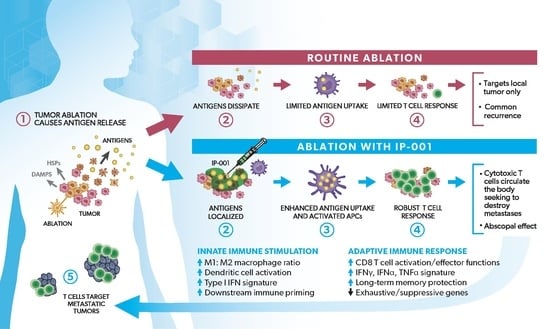
Novel Immune Stimulant Amplifies Direct Tumoricidal Effect of Cancer Ablation Therapies and Their Systemic Antitumor Immune Efficacy
Abstract
:1. Introduction
2. Tumor Ablation Therapies and Antitumor Immunity
- i.
- Rectifying changes in gene and protein expression at transcriptional and translational levels (best known are heat shock response, integrated stress response, unfolded protein response, and antioxidant response);
- ii.
- Cellular membrane repair responses engaging sterol regulatory element-binding proteins (SREBPs) pathway mediating the control of cholesterol and fatty acid metabolism and caspase-1 activity for maintaining cellular integrity (to be discussed below);
- iii.
- Improving disposal of damaged proteins and cells (including ERAD (ER-associated protein degradation response), autophagy, and various cell death signaling responses);
- iv.
- Inflammatory-immune and other cell non-autonomous responses (including DAMPs signaling, NF-κB activation signaling, Toll-like receptor (TLR) upregulation signaling, heat shock protein signaling, immunogenic cell death signaling, immunoregulatory cell signaling) [10].
3. The Family of N-Dihydrogalactochitosans as Promising Immune Stimulating Drugs
4. GC Combined with Thermal Ablation and Photodynamic Therapy
5. Potentiation by GC of Direct Tumoricidal Effect of Tumor Ablation Therapies
6. Clinical Application of GC
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barbari, C.; Fontaine, T.; Parajuli, P.; Lamichhane, N.; Jakubski, S.; Lamichane, P.; Deshmukh, R.R. Immunotherapies and Combination Strategies for immuno-oncology. Int. J. Mol. Sci. 2020, 21, 5009. [Google Scholar] [CrossRef]
- Knavel, E.M.; Brace, C.L. Tumor ablation: Common modalities and general practices. Tech. Vasc. Interr. Radiol. 2013, 16, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keisari, Y. Tumor abolition and antitumor immunostimulation by physico-chemical tumor ablation. Front. Biosci. Landmark 2017, 22, 310–347. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Fan, Y.-C.; Wang, K. Radiofrequency ablation versus microwave ablation for early stage hepatocellular carcinoma: A PRISMA-compliant system review and meta-analysis. Medicine 2020, 99, e22703. [Google Scholar] [CrossRef]
- Keisari, Y. Tumor Ablation. Effects on Systemic and Local Anti-Tumor Immunity and on Other Tumor-Microenvironment Interactions; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Ci, T.; Li, H.; Chen, G.; Wang, Z.; Wang, J.; Abdou, P.; Tu, Y.; Dotti, G.; Gu, Z. Cryo-shocked cancer cells for targeted drug delivery and vaccination. Sci. Adv. 2020, 6, eabc3013. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.; Girotti, A.; Gollnick, S.; Hahn, S.; Hamblin, M.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Balch, W.E.; Morimoto, R.I.; Dillin, A.; Kelly, J.W. Adapting proteostasis for diseases intervention. Science 2008, 318, 916–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, E.T.; Balch, W.E. Diversity in the origins of proteostasis networks—A driver for for protein function in evolution. Nat. Rev. Mol. Cell. Biol. 2013, 14, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M. Role of cell stress signaling networks in cancer cell death and antitumor immune response following proteotoxic injury inflicted by photodynamic therapy. Lasers Surg. Med. 2018, 50, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Starck, S.R.; Shastri, N. Nowhere to hide: Unconventional translation yields cryptic peptides for immune surveillance. Immunol. Rev. 2016, 271, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Zhou, F.; Nordquist, R.E.; Carubelli, R.; Liu, H.; Chen, W.R. Glycated chitosan as a new non-toxic immunological immunosimulant. Immunopharmacol. Immunotoxicol. 2009, 31, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Zhaou, F.; Wu, S.; Song, S.; Chen, W.R.; Resasco, D.E.; Xing, D. Antitumor immunologically modified carbon nanotube for photothermal therapy. Biomaterials 2012, 33, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Korbelik, M.; Bartels, K.E.; Liu, H.; Sun, J.; Nordquist, R.E. Enhancement of laser cancer treatment by chitosan-derived immunoadjuvant. Photochem. Photobiol. 2005, 81, 190–195. [Google Scholar] [CrossRef] [PubMed]
- El-Hussein, A.; Lam, S.S.; Raker, J.; Chen, W.R.; Hamblin, M.R. N-dihydrogalactochitosan as a potent immune activator of dendritic cells. J. Biomed. Mater. Res. A 2017, 105, 963–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korbelik, M.; Banáth, J.; Zhang, W.; Gallagher, P.; Hode, T.; Lam, S.S.K.; Chen, W.R. N-dihydrogalactochitosan as immune and direct antitumor agent amplifying the effects photodynamic therapy and photodynamic therapy-generated vaccines. Internat. Immunopharmacol. 2019, 75, 105764. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Wang, C.-Y.; Yang, F.-Y.; Wang, B.-S.; Chen, J.Y.; Lin, J.-D.; Leu, J.-D.; Chiu, S.-J.; Chen, F.-D.; Lee, Y.-J.; et al. Synergistic effects of glycated chitosan with high-intensity focused ultrasound on suppression of metastases in a syngeneic breast tumor model. Cell Death Dis. 2014, 5, e1178. [Google Scholar] [CrossRef] [Green Version]
- Korbelik, M.; Banáth, J.; Zhang, W.; Hode, T.; Lam, S.S.K.; Gallagher, P.; Zhao, J.; Zeng, H.; Chen, W.R. N-dihydrogalactochitosan-supported tumor control by photothermal therapy and photothermal therapy-generated vaccine. J. Photochem. Photobiol. B Biol. 2020, 204, 11780. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, X.; Naylor, M.F.; Hode, T.; Nordquist, R.E.; Alleruzzo, L.; Raker, J.; Lam, S.S.K.; Du, N.; Shi, L.; et al. InCVAX—A novel strategy for treatment of late-stage, metastatic cancers through photoimmunotherapy induced tumor-specific immunity. Cancer Lett. 2015, 359, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Yang, J.; Zhang, Y.; Liu, M.; Lang, M.L.; Li, M.; Chen, W.R. Local phototherapy synergizes with immunoadjuvant for treatment of pancreatic cancer through induced immunogenic tumor vaccine. Clin. Cancer Res. 2018, 24, 5335–5346. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Lam, S.S.K.; Liu, D.; Kim, D.Y.; Ma, L.; Alleruzzo, L.; Chen, W.; Hode, T.; Henry, C.J.; Kaifi, J.; et al. Development of inCVAX, in situ cancer vaccine, and its immune response in mice with hepatocellular cancer. J. Clin. Cell Immunol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Song, S.; Chen, W.R.; Xing, D. Immunostimulatory properties of glycated chitosan. J. Xray Sci. Technol. 2011, 19, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Zhu, W.-G.; Dynlacht, J.R.; Liu, H.; Nordquist, R.E. Long-term tumor resistance induced by laser photo-immunotherapy. Int. J. Cancer 1999, 81, 808–812. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Zhao, J.; Zeng, H.; Bielawska, A.; Szulc, Z.M. Mechanistic insights into ceramidase inhibitor LCL521-enhanced tumor cell kill by photodynamic and thermal ablation therapies. Photochem. Photobiol. Sci. 2020, 19, 1145–1151. [Google Scholar] [CrossRef]
- Emeagi, P.U.; Maenhout, S.; Dang, N.; Thielemans, K.; Breckpot, K. Downregulation of Stat3 in melanoma: Reprogramming the immune environment as an cancer therapy strategy. Gene Ther. 2013, 20, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Lizarbe, M.A.; Barrasa, J.I.; Olmo, N.; Gavilanes, F.; Turnay, J. Annexin-phospholipid interactions. Functional implications. Int. J. Mol. Sci. 2013, 14, 2652–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korbelik, M.; Banáth, J.; Saw, K.M.; Zhang, W.; Čyplys, E. Calreticulin as cancer treatment adjuvant: Combination with photodynamic therapy and photodynamic therapy-generated vaccines. Front. Oncol. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Merchant, S.; Korbelik, M. Heat shock protein 70 is acute phase reactant: Response elicited by tumor treatment with photodynamic therapy. Cell Stress Chaperones 2011, 16, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, D.; Vince, J.E. Pyroptosis versus necroptosis: Similarities differences, and crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef]
- Pei, Y.; Geng, T.; Su, L. Pyroptosis of HUVECS can be induced by heat stroke. Biochem. Biophys. Res. Commun. 2018, 506, 626–631. [Google Scholar] [CrossRef]
- Sollberger, G.; Strittmatter, G.E.; Garstkiewitz, M.; Sand, J.; Beer, H.-D. Caspase-1: The inflammasome and beyond. Innate Immun. 2014, 20, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurcel, L.; Abrami, L.; Girardin, S.; Tschopp, J.; van der Goot, F.G. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 2006, 126, 1135–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology—Divergent pathophysiology. Nat Rev Endocrinol 2017, 13, 710–730. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ferrel, G.L.; Guerra, M.C.; Hode, T.; Lunn, J.A.; Adalsteinsson, O.; Nordquist, R.E.; Liu, H.; Chen, W.R. Preliminary safety and efficacy results of laser immunotherapy for the treatment of metastatic breast cancer patients. Photochem. Photobiol. Sci. 2011, 10, 817–821. [Google Scholar] [CrossRef] [PubMed]

| Patient # | Age | AJCC Stage | ER | PR | HER2/Neu | Surgery | Chemo | Radiation Therapy | Hormonal Therapy | Best Overall Response |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | III | + | + | + | No | Yes | Yes | Yes | NE |
| 2 | 47 | IV | + | + | − | No | Yes | No | Yes | CR |
| 3 | 43 | III | − | − | − | No | Yes | Yes | No | PD |
| 4 | 36 | III | + | + | - | No | Yes | Yes | No | NE |
| 5 | 40 | IV | − | + | + | Yes | Yes | Yes | Yes | PR |
| 6 | 85 | IV | − | − | − | No | No | No | No | PR |
| 7 | 78 | III | Unk | Unk | Unk | No | No | No | No | PR |
| 8 | 58 | III | − | − | − | No | No | No | No | PD |
| 9 | 66 | IV | + | + | Unk | Yes | Yes | Yes | Yes | PR |
| 10 | 39 | IV | − | + | − | Yes | Yes | Yes | Yes | SD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korbelik, M.; Hode, T.; Lam, S.S.K.; Chen, W.R. Novel Immune Stimulant Amplifies Direct Tumoricidal Effect of Cancer Ablation Therapies and Their Systemic Antitumor Immune Efficacy. Cells 2021, 10, 492. https://doi.org/10.3390/cells10030492
Korbelik M, Hode T, Lam SSK, Chen WR. Novel Immune Stimulant Amplifies Direct Tumoricidal Effect of Cancer Ablation Therapies and Their Systemic Antitumor Immune Efficacy. Cells. 2021; 10(3):492. https://doi.org/10.3390/cells10030492
Chicago/Turabian StyleKorbelik, Mladen, Tomas Hode, Samuel S. K. Lam, and Wei R. Chen. 2021. "Novel Immune Stimulant Amplifies Direct Tumoricidal Effect of Cancer Ablation Therapies and Their Systemic Antitumor Immune Efficacy" Cells 10, no. 3: 492. https://doi.org/10.3390/cells10030492
APA StyleKorbelik, M., Hode, T., Lam, S. S. K., & Chen, W. R. (2021). Novel Immune Stimulant Amplifies Direct Tumoricidal Effect of Cancer Ablation Therapies and Their Systemic Antitumor Immune Efficacy. Cells, 10(3), 492. https://doi.org/10.3390/cells10030492