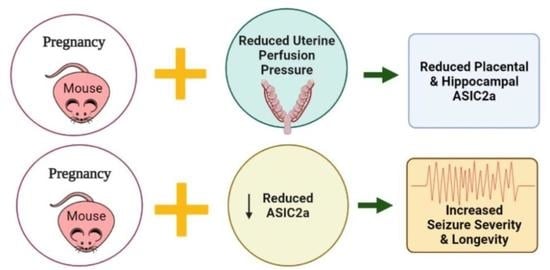
Acid Sensing Ion Channel 2a Is Reduced in the Reduced Uterine Perfusion Pressure Mouse Model and Increases Seizure Susceptibility in Pregnant Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Breeding and Establishment of Timed Pregnancy for ASIC2a Mice
2.3. Mouse RUPP Model
2.4. Euthanasia and Tissue Collection
2.5. ELISA Analysis
2.6. Western Blot Analysis
2.7. Measurement of Hypoxia-Inducible Factor in Placental Samples
2.8. Seizure Induction Procedure
2.9. ASIC2a Genotyping
2.10. Data Analysis
3. Results
3.1. The Mouse RUPP Model Has Some Similar Clinical Characteristics to the Preeclampsia Patient and RUPP Rat
3.2. The RUPP Model in the Mouse Induced Reduced ASIC2a Protein Expression in the Placenta and Hippocampus
3.3. General Characteristics of Pregnant ASIC2a Wild-Type and Heterozygous Knockout Mice at GD18.5
3.4. Knockdown of ASIC2a Results in Increased Seizure Severity and Longevity during Late Pregnancy
4. Discussion
4.1. The Mouse RUPP Model
4.2. Reduced ASIC2a Expression
4.3. Seizures in Pregnant ASIC2a Mice
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fingar, K.R.; Mabry-Hernandez, I.; Ngo-Metzger, Q.; Wolff, T.; Steiner, C.A.; Elixhauser, A. Delivery Hospitalization Involving Preeclampsia and Eclampsia, 2005–2014. HCUP Statistical Brief #0222. April 2017. Agency for Healthcare Research and Quality, Rockville, MD. Available online: www.hcup-us.ahrq.gov/reports/statbriefs/sb222-Preeclampsia-Eclampsia-Delivery-Trends.pdf (accessed on 19 November 2019).
- Vousden, N.; Lawley, E.; Seed, P.T.; Gidiri, M.F.; Goudar, S.; Sandall, J.; Chappell, L.C.; Shennan, A.H.; Group, C.T.C. Incidence of eclampsia and related complications across 10 low- and middle-resource geographical regions: Secondary analysis of a cluster randomised controlled trial. PLoS Med. 2019, 16, e1002775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghulmiyyah, L.; Sibai, B. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 2012, 36, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.M. Diagnosis, prevention, and management of eclampsia. Obstet. Gynecol. 2005, 105, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Ijomone, O.K.; Shallie, P.; Naicker, T. Changes in the structure and function of the brain years after Pre-eclampsia. Ageing Res. Rev. 2018, 47, 49–54. [Google Scholar] [CrossRef]
- Goffin, S.M.; Derraik, J.G.B.; Groom, K.M.; Cutfield, W.S. Maternal pre-eclampsia and long-term offspring health: Is there a shadow cast? Pregnancy Hypertens. 2018, 12, 11–15. [Google Scholar] [CrossRef]
- Dang, F.; Croy, B.A.; Stroman, P.W.; Figueiro-Filho, E.A. Impacts of Preeclampsia on the Brain of the Offspring. Rev. Bras. Ginecol. Obstet. 2016, 38, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Office of Communications. What Are the Treatments for Preeclampsia, Eclampsia, & HELLP Syndrome? Available online: https://www.nichd.nih.gov/health/topics/preeclampsia/conditioninfo/treatments (accessed on 29 April 2020).
- Lubarsky, S.L.; Barton, J.R.; Friedman, S.A.; Nasreddine, S.; Ramadan, M.K.; Sibai, B.M. Late postpartum eclampsia revisited. Obstet. Gynecol. 1994, 83, 502–505. [Google Scholar] [CrossRef]
- Duley, L.; Gulmezoglu, A.M.; Henderson-Smart, D.J.; Chou, D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst. Rev. 2010, 11, CD000025. [Google Scholar] [CrossRef]
- Huang, R.Q.; Bell-Horner, C.L.; Dibas, M.I.; Covey, D.F.; Drewe, J.A.; Dillon, G.H. Pentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: Mechanism and site of action. J. Pharmacol. Exp. Ther. 2001, 298, 986–995. [Google Scholar]
- Huang, Q.; Liu, L.; Hu, B.; Di, X.; Brennecke, S.P.; Liu, H. Decreased seizure threshold in an eclampsia-like model induced in pregnant rats with lipopolysaccharide and pentylenetetrazol treatments. PLoS ONE 2014, 9, e89333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warrington, J.P. Placental ischemia increases seizure susceptibility and cerebrospinal fluid cytokines. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef]
- Johnson, A.C.; Nagle, K.J.; Tremble, S.M.; Cipolla, M.J. The Contribution of Normal Pregnancy to Eclampsia. PLoS ONE 2015, 10, e0133953. [Google Scholar] [CrossRef]
- Boscardin, E.; Alijevic, O.; Hummler, E.; Frateschi, S.; Kellenberger, S. The function and regulation of acid-sensing ion channels (ASICs) and the epithelial Na(+) channel (ENaC): IUPHAR Review 19. Br. J. Pharmacol. 2016, 173, 2671–2701. [Google Scholar] [CrossRef]
- Wu, H.; Wang, C.; Liu, B.; Li, H.; Zhang, Y.; Dong, S.; Gao, G.; Zhang, H. Altered Expression Pattern of Acid-Sensing Ion Channel Isoforms in Piriform Cortex After Seizures. Mol. Neurobiol. 2016, 53, 1782–1793. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, G.; Zhang, Y.; Sun, Y.; Li, H.; Dong, S.; Ma, W.; Liu, B.; Wang, W.; Wu, H.; et al. Glucose Deficiency Elevates Acid-Sensing Ion Channel 2a Expression and Increases Seizure Susceptibility in Temporal Lobe Epilepsy. Sci. Rep. 2017, 7, 5870. [Google Scholar] [CrossRef] [Green Version]
- Price, M.P.; Lewin, G.R.; McIlwrath, S.L.; Cheng, C.; Xie, J.; Heppenstall, P.A.; Stucky, C.L.; Mannsfeldt, A.G.; Brennan, T.J.; Drummond, H.A.; et al. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 2000, 407, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Fushima, T.; Sekimoto, A.; Minato, T.; Ito, T.; Oe, Y.; Kisu, K.; Sato, E.; Funamoto, K.; Hayase, T.; Kimura, Y.; et al. Reduced Uterine Perfusion Pressure (RUPP) Model of Preeclampsia in Mice. PLoS ONE 2016, 11, e0155426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, C.; Barer, G.R.; Shaw, J.W.; Clegg, E.J. Growth of the heart and lungs in hypoxic rodents: A model of human hypoxic disease. Clin. Sci. Mol. Med. 1974, 46, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Luttjohann, A.; Fabene, P.F.; van Luijtelaar, G. A revised Racine’s scale for PTZ-induced seizures in rats. Physiol. Behav. 2009, 98, 579–586. [Google Scholar] [CrossRef]
- Li, J.; LaMarca, B.; Reckelhoff, J.F. A model of preeclampsia in rats: The reduced uterine perfusion pressure (RUPP) model. Am. J. Physiol. Heart C 2012, 303, H1–H8. [Google Scholar] [CrossRef]
- Morton, J.S.; Levasseur, J.; Ganguly, E.; Quon, A.; Kirschenman, R.; Dyck, J.R.B.; Fraser, G.M.; Davidge, S.T. Characterisation of the Selective Reduced Uteroplacental Perfusion (sRUPP) Model of Preeclampsia. Sci. Rep. 2019, 9, 9565. [Google Scholar] [CrossRef]
- Gilbert, J.S.; Babcock, S.A.; Granger, J.P. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 2007, 50, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Granger, J.P.; LaMarca, B.B.; Cockrell, K.; Sedeek, M.; Balzi, C.; Chandler, D.; Bennett, W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol. Med. 2006, 122, 383–392. [Google Scholar] [CrossRef]
- Helske, S.; Vuorela, P.; Carpen, O.; Hornig, C.; Weich, H.; Halmesmaki, E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol. Hum. Reprod. 2001, 7, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madazli, R.; Aydin, S.; Uludag, S.; Vildan, O.; Tolun, N. Maternal plasma levels of cytokines in normal and preeclamptic pregnancies and their relationship with diastolic blood pressure and fibronectin levels. Acta Obstet. Gynecol. Scand. 2003, 82, 797–802. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intapad, S.; Warrington, J.P.; Spradley, F.T.; Palei, A.C.; Drummond, H.A.; Ryan, M.J.; Granger, J.P.; Alexander, B.T. Reduced uterine perfusion pressure induces hypertension in the pregnant mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1353–R1357. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, P.K.; Jain, V.; Sakhuja, V.; Karumanchi, S.A.; Jha, V. Low urinary placental growth factor is a marker of pre-eclampsia. Kidney Int 2006, 69, 621–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingston, J.C.; Chin, R.; Haddad, B.; McKinney, E.T.; Ahokas, R.; Sibai, B.M. Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe preeclampsia. Am. J. Obstet. Gynecol. 2000, 183, 1554–1557. [Google Scholar] [CrossRef]
- Levine, R.J.; Karumanchi, S.A. Circulating angiogenic factors in preeclampsia. Clin. Obstet. Gynecol. 2005, 48, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.N.; Grimwood, J.; Taylor, R.S.; McMaster, M.T.; Fisher, S.J.; North, R.A. Longitudinal serum concentrations of placental growth factor: Evidence for abnormal placental angiogenesis in pathologic pregnancies. Am. J. Obstet. Gynecol. 2003, 188, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.J.; Suh, B.C. Acid-sensing ion channels (ASICs): Therapeutic targets for neurological diseases and their regulation. BMB Rep. 2013, 46, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.J.; Huang, L.F.; Chen, X.M.; Pan, S.Q.; Lu, Z.N.; Xiao, Z.M. Amiloride suppresses pilocarpine-induced seizures via ASICs other than NHE in rats. Int. J. Clin. Exp. Pathol. 2015, 8, 14507–14513. [Google Scholar] [PubMed]
- Cristofori-Armstrong, B.; Rash, L.D. Acid-sensing ion channel (ASIC) structure and function: Insights from spider, snake and sea anemone venoms. Neuropharmacology 2017, 127, 173–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Q.; Wang, W.; Gu, J.; Jiang, G.; Wang, K.; Xu, Z.; Li, J.; Chen, G.; Wang, X. Elevated Expression of Acid-Sensing Ion Channel 3 Inhibits Epilepsy via Activation of Interneurons. Mol. Neurobiol. 2016, 53, 485–498. [Google Scholar] [CrossRef]
- Yang, F.; Sun, X.; Ding, Y.; Ma, H.; Yang, T.O.; Ma, Y.; Wei, D.; Li, W.; Xu, T.; Jiang, W. Astrocytic Acid-Sensing Ion Channel 1a Contributes to the Development of Chronic Epileptogenesis. Sci. Rep. 2016, 6, 31581. [Google Scholar] [CrossRef]
- Yermolaieva, O.; Leonard, A.S.; Schnizler, M.K.; Abboud, F.M.; Welsh, M.J. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA 2004, 101, 6752–6757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayan, N.; Kaur, A.; Elharram, M.; Rossi, A.M.; Pilote, L. Impact of Preeclampsia on Long-Term Cognitive Function. Hypertension 2018, 72, 1374–1380. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chiou, H.Y.; Lin, H.C.; Lin, H.L. Affect of seizures during gestation on pregnancy outcomes in women with epilepsy. Arch. Neurol. 2009, 66, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, T.Y.; Broughton Pipkin, F.; Khan, R.N. The effect of pH and ion channel modulators on human placental arteries. PLoS ONE 2014, 9, e114405. [Google Scholar] [CrossRef] [PubMed]
- Hanukoglu, I. ASIC and ENaC type sodium channels: Conformational states and the structures of the ion selectivity filters. FEBS J. 2017, 284, 525–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meltzer, R.H.; Kapoor, N.; Qadri, Y.J.; Anderson, S.J.; Fuller, C.M.; Benos, D.J. Heteromeric assembly of acid-sensitive ion channel and epithelial sodium channel subunits. J. Biol. Chem. 2007, 282, 25548–25559. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Long, X.; Arends, J.; Aarts, R.M. EEG analysis of seizure patterns using visibility graphs for detection of generalized seizures. J. Neurosci. Methods 2017, 290, 85–94. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Sham (n = 6) | RUPP (n = 6) | p-Value |
|---|---|---|---|
| Maternal | |||
| Body weight (g) | 39.2 ± 1.4 | 37.8 ± 1.9 | 0.289 |
| Hematocrit (%) | 35 ± 2 | 35 ± 1 | ‡ 0.446 |
| Fetal Outcomes | |||
| No. of live pups | 8 ± 1 | 5 ± 1 | 0.0499 † |
| % resorptions | 0 ± 0 | 21 ± 15 | 0.111 |
| Pup weight (g) | 1.13 ± 0.03 | 1.21 ± 0.06 | § 0.294 |
| Placenta weight (g) | 0.09 ± 0.00 | 0.10 ± 0.00 | 0.173 |
| Pup Hematocrit (%) | 30 ± 2 | 35 ± 1 | 0.037 † |
| Characteristic | ASIC2a+/+ (n = 7) | ASIC2a+/− (n = 14) | p-Value |
|---|---|---|---|
| Maternal | |||
| Body weight (g) | 35.5 ± 2.2 | 35.4±3.0 | 0.406 |
| Fetal Outcomes | |||
| No. of live pups | 8 ± 1 | 7 ± 2 | 0.358 |
| % resorptions | 11 ± 13 | 5 ± 6 | 0.071 |
| Pup weight (g) | 0.87 ± 0.02 | 0.85 ± 0.01 | 0.096 |
| Placenta weight (g) | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.066 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones-Muhammad, M.; Shao, Q.; Cain-Shields, L.; Shaffery, J.P.; Warrington, J.P. Acid Sensing Ion Channel 2a Is Reduced in the Reduced Uterine Perfusion Pressure Mouse Model and Increases Seizure Susceptibility in Pregnant Mice. Cells 2021, 10, 1135. https://doi.org/10.3390/cells10051135
Jones-Muhammad M, Shao Q, Cain-Shields L, Shaffery JP, Warrington JP. Acid Sensing Ion Channel 2a Is Reduced in the Reduced Uterine Perfusion Pressure Mouse Model and Increases Seizure Susceptibility in Pregnant Mice. Cells. 2021; 10(5):1135. https://doi.org/10.3390/cells10051135
Chicago/Turabian StyleJones-Muhammad, Maria, Qingmei Shao, Loretta Cain-Shields, James P. Shaffery, and Junie P. Warrington. 2021. "Acid Sensing Ion Channel 2a Is Reduced in the Reduced Uterine Perfusion Pressure Mouse Model and Increases Seizure Susceptibility in Pregnant Mice" Cells 10, no. 5: 1135. https://doi.org/10.3390/cells10051135
APA StyleJones-Muhammad, M., Shao, Q., Cain-Shields, L., Shaffery, J. P., & Warrington, J. P. (2021). Acid Sensing Ion Channel 2a Is Reduced in the Reduced Uterine Perfusion Pressure Mouse Model and Increases Seizure Susceptibility in Pregnant Mice. Cells, 10(5), 1135. https://doi.org/10.3390/cells10051135