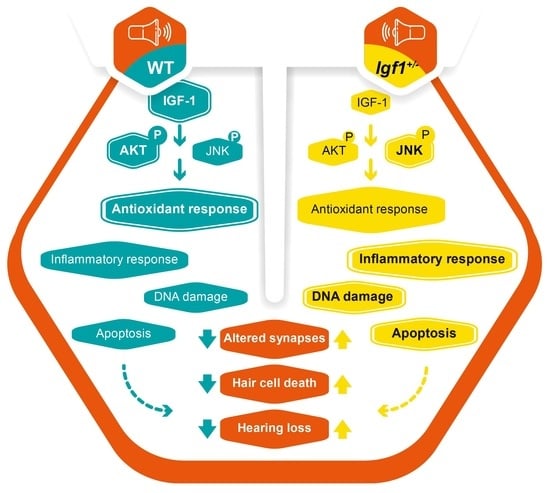
IGF-1 Haploinsufficiency Causes Age-Related Chronic Cochlear Inflammation and Increases Noise-Induced Hearing Loss
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Comparative IGF-System and Cytokines Gene Expression Profiling, IGF-1 Serum Levels, and Hearing Thresholds of Igf1+/− and WT Mice along Age
3.2. Adult Igf1+/− Mice Show Increased Susceptibility to Noise Injury
3.3. Age-Related and Noise-Induced Cochlear Antioxidant Gene Expression Profiling of Igf1+/− and WT Mice
3.4. Cochlear Noise-Induced Inflammatory Response and Cell Death Are Exacerbated in Adult Igf1+/− Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Report on Hearing. Available online: https://www.who.int/publications-detail-redirect/world-report-on-hearing (accessed on 4 June 2021).
- Bowl, M.R.; Brown, S.D.M. Genetic Landscape of Auditory Dysfunction. Hum. Mol. Genet. 2018, 27, R130–R135. [Google Scholar] [CrossRef]
- Wesdorp, M.; Murillo-Cuesta, S.; Peters, T.; Celaya, A.M.; Oonk, A.; Schraders, M.; Oostrik, J.; Gomez-Rosas, E.; Beynon, A.J.; Hartel, B.P.; et al. MPZL2, Encoding the Epithelial Junctional Protein Myelin Protein Zero-like 2, Is Essential for Hearing in Man and Mouse. Am. J. Hum. Genet. 2018, 103, 74–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela-Nieto, I.; Murillo-Cuesta, S.; Rodríguez-de la Rosa, L.; Lassatetta, L.; Contreras, J. IGF-I Deficiency and Hearing Loss: Molecular Clues and Clinical Implications. Pediatr. Endocrinol. Rev. 2013, 10, 460–472. [Google Scholar] [PubMed]
- Batey, L.; Moon, J.E.; Yu, Y.; Wu, B.; Hirschhorn, J.N.; Shen, Y.; Dauber, A. A Novel Deletion of IGF1 in a Patient with Idiopathic Short Stature Provides Insight Into IGF1 Haploinsufficiency. J. Clin. Endocrinol. Metab. 2014, 99, E153–E159. [Google Scholar] [CrossRef]
- Fuqua, J.S.; Derr, M.; Rosenfeld, R.G.; Hwa, V. Identification of a Novel Heterozygous IGF1 Splicing Mutation in a Large Kindred with Familial Short Stature. Horm. Res. Paediatr. 2012, 78, 59–66. [Google Scholar] [CrossRef] [PubMed]
- van Duyvenvoorde, H.A.; van Setten, P.A.; Walenkamp, M.J.E.; van Doorn, J.; Koenig, J.; Gauguin, L.; Oostdijk, W.; Ruivenkamp, C.A.L.; Losekoot, M.; Wade, J.D.; et al. Short Stature Associated with a Novel Heterozygous Mutation in the Insulin-like Growth Factor 1 Gene. J. Clin. Endocrinol. Metab. 2010, 95, E363–E367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnard, Å.; Bark, R.; Hederstierna, C. Clinical Update on Sensorineural Hearing Loss in Turner Syndrome and the X-Chromosome. Am. J. Med. Genet. C Semin. Med. Genet. 2019, 181, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Attias, J.; Zarchi, O.; Nageris, B.I.; Laron, Z. Cochlear Hearing Loss in Patients with Laron Syndrome. Eur. Arch. Otorhinolaryngol. 2012, 269, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E.; Allanson, J.E.; Tartaglia, M.; Gelb, B.D. Noonan Syndrome. Lancet 2013, 381, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-de la Rosa, L.; Lassaletta, L.; Calvino, M.; Murillo-Cuesta, S.; Varela-Nieto, I. The Role of Insulin-Like Growth Factor 1 in the Progression of Age-Related Hearing Loss. Front. Aging Neurosci. 2017, 9, 411. [Google Scholar] [CrossRef] [Green Version]
- Gómez, J.-M. Serum Leptin, Insulin-like Growth Factor-I Components and Sex-Hormone Binding Globulin. Relationship with Sex, Age and Body Composition in Healthy Population. Protein Pept. Lett. 2007, 14, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa, N.; Vendrell, J.; Maravall, J.; Broch, M.; Estepa, A.; Megia, A.; Soler, J.; Simón, I.; Richart, C.; Gómez, J.M. Distribution and Determinants of Adiponectin, Resistin and Ghrelin in a Randomly Selected Healthy Population. Clin. Endocrinol. (Oxf.) 2005, 63, 329–335. [Google Scholar] [CrossRef]
- Cano, A.; Dargent, G.; Carriazo, A.; López-Samaniego, L.; Apostolo, J.; Campos, E.; Holland, C.; Varela-Nieto, I.; Luz Sánchez-Sánchez, M.; Illario, M.; et al. Tackling Frailty and Functional Decline: Background of the Action Group A3 of the European Innovation Partnership for Active and Healthy Ageing. Maturitas 2018, 115, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pharaoh, G.; Owen, D.; Yeganeh, A.; Premkumar, P.; Farley, J.; Bhaskaran, S.; Ashpole, N.; Kinter, M.; Van Remmen, H.; Logan, S. Disparate Central and Peripheral Effects of Circulating IGF-1 Deficiency on Tissue Mitochondrial Function. Mol. Neurobiol. 2020, 57, 1317–1331. [Google Scholar] [CrossRef] [Green Version]
- Varela-Nieto, I.; Murillo-Cuesta, S.; Calvino, M.; Cediel, R.; Lassaletta, L. Drug Development for Noise-Induced Hearing Loss. Expert. Opin. Drug Discov. 2020, 15, 1457–1471. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C. Noise-Induced and Age-Related Hearing Loss: New Perspectives and Potential Therapies. F1000Res 2017, 6, 927. [Google Scholar] [CrossRef]
- Wang, J.; Puel, J.-L. Presbycusis: An Update on Cochlear Mechanisms and Therapies. J. Clin. Med. 2020, 9, 218. [Google Scholar] [CrossRef] [Green Version]
- Celaya, A.M.; Sánchez-Pérez, I.; Bermúdez-Muñoz, J.M.; Rodríguez-de la Rosa, L.; Pintado-Berninches, L.; Perona, R.; Murillo-Cuesta, S.; Varela-Nieto, I. Deficit of Mitogen-Activated Protein Kinase Phosphatase 1 (DUSP1) Accelerates Progressive Hearing Loss. eLife 2019, 8. [Google Scholar] [CrossRef]
- Perin, P.; Marino, F.; Varela-Nieto, I.; Szczepek, A.J. Editorial: Neuroimmunology of the Inner Ear. Front. Neurol. 2021, 12, 635359. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Nakagawa, T.; Endo, T.; Matsuoka, Y.; Kita, T.; Kim, T.-S.; Tabata, Y.; Ito, J. Cochlear Protection by Local Insulin-like Growth Factor-1 Application Using Biodegradable Hydrogel. Laryngoscope 2006, 116, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Nakagawa, T.; Okano, T.; Hori, R.; Ono, K.; Tabata, Y.; Lee, S.H.; Ito, J. Novel Therapy for Hearing Loss: Delivery of Insulin-like Growth Factor 1 to the Cochlea Using Gelatin Hydrogel. Otol. Neurotol. 2007, 28, 976–981. [Google Scholar] [CrossRef]
- Fujiwara, T.; Hato, N.; Nakagawa, T.; Tabata, Y.; Yoshida, T.; Komobuchi, H.; Takeda, S.; Hyodo, J.; Hakuba, N.; Gyo, K. Insulin-like Growth Factor 1 Treatment via Hydrogels Rescues Cochlear Hair Cells from Ischemic Injury. Neuroreport 2008, 19, 1585–1588. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Kita, T.; Katsuno, T.; Yamamoto, N.; Omori, K.; Nakagawa, T. Insulin-Like Growth Factor 1 on the Maintenance of Ribbon Synapses in Mouse Cochlear Explant Cultures. Front. Cell Neurosci. 2020, 14, 571155. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Costa-Besada, M.A.; Labandeira, C.M.; Villar-Cheda, B.; Rodríguez-Perez, A.I. Insulin-Like Growth Factor-1 and Neuroinflammation. Front. Aging Neurosci. 2017, 9, 365. [Google Scholar] [CrossRef]
- Smith, T.J. Insulin-like Growth Factor-I Regulation of Immune Function: A Potential Therapeutic Target in Autoimmune Diseases? Pharmacol. Rev. 2010, 62, 199–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, J.C.; McCusker, R.H.; Strle, K.; Johnson, R.W.; Dantzer, R.; Kelley, K.W. Regulation of IGF-I Function by Proinflammatory Cytokines: At the Interface of Immunology and Endocrinology. Cell Immunol. 2008, 252, 91–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, F.; Muderris, T.; Yalciner, G.; Sevil, E.; Bercin, S.; Ergin, M.; Babademez, M.A.; Kiris, M. A Comprehensive Study of Oxidative Stress in Sudden Hearing Loss. Eur. Arch. Otorhinolaryngol. 2017, 274, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Kumakawa, K.; Usami, S.; Hato, N.; Tabuchi, K.; Takahashi, M.; Fujiwara, K.; Sasaki, A.; Komune, S.; Sakamoto, T.; et al. A Randomized Controlled Clinical Trial of Topical Insulin-like Growth Factor-1 Therapy for Sudden Deafness Refractory to Systemic Corticosteroid Treatment. BMC Med. 2014, 12, 219. [Google Scholar] [CrossRef] [Green Version]
- Yamahara, K.; Yamamoto, N.; Nakagawa, T.; Ito, J. Insulin-like Growth Factor 1: A Novel Treatment for the Protection or Regeneration of Cochlear Hair Cells. Hear. Res. 2015, 330, 2–9. [Google Scholar] [CrossRef]
- Liu, J.P.; Baker, J.; Perkins, A.S.; Robertson, E.J.; Efstratiadis, A. Mice Carrying Null Mutations of the Genes Encoding Insulin-like Growth Factor I (Igf-1) and Type 1 IGF Receptor (Igf1r). Cell 1993, 75, 59–72. [Google Scholar] [CrossRef]
- Sanchez-Calderon, H.; Rodriguez-de la Rosa, L.; Milo, M.; Pichel, J.G.; Holley, M.; Varela-Nieto, I. RNA Microarray Analysis in Prenatal Mouse Cochlea Reveals Novel IGF-I Target Genes: Implication of MEF2 and FOXM1 Transcription Factors. PLoS ONE 2010, 5, e8699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cediel, R.; Riquelme, R.; Contreras, J.; Díaz, A.; Varela-Nieto, I. Sensorineural Hearing Loss in Insulin-like Growth Factor I-Null Mice: A New Model of Human Deafness. Eur. J. Neurosci. 2006, 23, 587–590. [Google Scholar] [CrossRef]
- Sanz, L.; Murillo-Cuesta, S.; Cobo, P.; Cediel-Algovia, R.; Contreras, J.; Rivera, T.; Varela-Nieto, I.; Avendaño, C. Swept-Sine Noise-Induced Damage as a Hearing Loss Model for Preclinical Assays. Front. Aging Neurosci. 2015, 7, 7. [Google Scholar] [CrossRef]
- Murillo-Cuesta, S.; Rodríguez-de la Rosa, L.; Contreras, J.; Celaya, A.M.; Camarero, G.; Rivera, T.; Varela-Nieto, I. Transforming Growth Factor Β1 Inhibition Protects from Noise-Induced Hearing Loss. Front. Aging Neurosci. 2015, 7, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partearroyo, T.; Murillo-Cuesta, S.; Vallecillo, N.; Bermúdez-Muñoz, J.M.; Rodríguez-de la Rosa, L.; Mandruzzato, G.; Celaya, A.M.; Zeisel, S.H.; Pajares, M.A.; Varela-Moreiras, G.; et al. Betaine-Homocysteine S-Methyltransferase Deficiency Causes Increased Susceptibility to Noise-Induced Hearing Loss Associated with Plasma Hyperhomocysteinemia. FASEB J. 2019, 33, 5942–5956. [Google Scholar] [CrossRef] [PubMed]
- de Iriarte Rodríguez, R.; Magariños, M.; Pfeiffer, V.; Rapp, U.R.; Varela-Nieto, I. C-Raf Deficiency Leads to Hearing Loss and Increased Noise Susceptibility. Cell Mol. Life Sci. 2015, 72, 3983–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camarero, G.; Avendano, C.; Fernandez-Moreno, C.; Villar, A.; Contreras, J.; de Pablo, F.; Pichel, J.G.; Varela-Nieto, I. Delayed Inner Ear Maturation and Neuronal Loss in Postnatal Igf-1-Deficient Mice. J. Neurosci. 2001, 21, 7630–7641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riquelme, R.; Cediel, R.; Contreras, J.; la Rosa Lourdes, R.; Murillo-Cuesta, S.; Hernandez-Sanchez, C.; Zubeldia, J.M.; Cerdan, S.; Varela-Nieto, I. A Comparative Study of Age-Related Hearing Loss in Wild Type and Insulin-like Growth Factor I Deficient Mice. Front. Neuroanat. 2010, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Espino Guarch, M.; Font-Llitjós, M.; Murillo-Cuesta, S.; Errasti-Murugarren, E.; Celaya, A.M.; Girotto, G.; Vuckovic, D.; Mezzavilla, M.; Vilches, C.; Bodoy, S.; et al. Mutations in L-Type Amino Acid Transporter-2 Support SLC7A8 as a Novel Gene Involved in Age-Related Hearing Loss. eLife 2018, 7. [Google Scholar] [CrossRef]
- Saper, C.B. Unbiased Stereology: Three-Dimensional Measurement in Microscopy by C.V. Howard and M.G. Reed. Trends Neurosci. 1999, 22, 94–95. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Camarero, G.; Villar, M.A.; Contreras, J.; Fernández-Moreno, C.; Pichel, J.G.; Avendaño, C.; Varela-Nieto, I. Cochlear Abnormalities in Insulin-like Growth Factor-1 Mouse Mutants. Hear. Res. 2002, 170, 2–11. [Google Scholar] [CrossRef]
- Partearroyo, T.; Vallecillo, N.; Pajares, M.A.; Varela-Moreiras, G.; Varela-Nieto, I. Cochlear Homocysteine Metabolism at the Crossroad of Nutrition and Sensorineural Hearing Loss. Front. Mol. Neurosci. 2017, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermúdez-Muñoz, J.M.; Celaya, A.M.; Hijazo-Pechero, S.; Wang, J.; Serrano, M.; Varela-Nieto, I. G6PD Overexpression Protects from Oxidative Stress and Age-Related Hearing Loss. Aging Cell 2020, 19, e13275. [Google Scholar] [CrossRef]
- Gong, Z.; Kennedy, O.; Sun, H.; Wu, Y.; Williams, G.A.; Klein, L.; Cardoso, L.; Matheny, R.W.; Hubbard, G.B.; Ikeno, Y.; et al. Reductions in Serum IGF-1 during Aging Impair Health Span. Aging Cell 2014, 13, 408–418. [Google Scholar] [CrossRef]
- Rodriguez-de la Rosa, L.; Fernandez-Sanchez, L.; Germain, F.; Murillo-Cuesta, S.; Varela-Nieto, I.; de la Villa, P.; Cuenca, N. Age-Related Functional and Structural Retinal Modifications in the Igf1−/− Null Mouse. Neurobiol. Dis. 2012, 46, 476–485. [Google Scholar] [CrossRef]
- Yamahara, K.; Asaka, N.; Kita, T.; Kishimoto, I.; Matsunaga, M.; Yamamoto, N.; Omori, K.; Nakagawa, T. Insulin-like Growth Factor 1 Promotes Cochlear Synapse Regeneration after Excitotoxic Trauma in Vitro. Hear. Res. 2019, 374, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Skoe, E.; Tufts, J. Evidence of Noise-Induced Subclinical Hearing Loss Using Auditory Brainstem Responses and Objective Measures of Noise Exposure in Humans. Hear. Res. 2018, 361, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Santamaría, V.; Alvarado, J.C.; Rodríguez-de la Rosa, L.; Murillo-Cuesta, S.; Contreras, J.; Juiz, J.M.; Varela-Nieto, I. IGF-1 Deficiency Causes Atrophic Changes Associated with Upregulation of VGluT1 and Downregulation of MEF2 Transcription Factors in the Mouse Cochlear Nuclei. Brain Struct. Funct. 2016, 221, 709–734. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Santamaría, V.; Alvarado, J.C.; Rodríguez-de la Rosa, L.; Juiz, J.M.; Varela-Nieto, I. Neuroglial Involvement in Abnormal Glutamate Transport in the Cochlear Nuclei of the Igf1−/− Mouse. Front. Cell Neurosci. 2019, 13, 67. [Google Scholar] [CrossRef] [Green Version]
- Maison, S.F.; Usubuchi, H.; Liberman, M.C. Efferent Feedback Minimizes Cochlear Neuropathy from Moderate Noise Exposure. J. Neurosci. 2013, 33, 5542–5552. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hirose, K.; Liberman, M.C. Dynamics of Noise-Induced Cellular Injury and Repair in the Mouse Cochlea. J. Assoc. Res. Otolaryngol. 2002, 3, 248–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, Y.; Yamamoto, N.; Nakagawa, T.; Ito, J. Insulin-like Growth Factor 1 Inhibits Hair Cell Apoptosis and Promotes the Cell Cycle of Supporting Cells by Activating Different Downstream Cascades after Pharmacological Hair Cell Injury in Neonatal Mice. Mol. Cell Neurosci. 2013, 56, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, Y.; Yamamoto, N.; Nakagawa, T.; Ito, J. Insulin-like Growth Factor 1 Induces the Transcription of Gap43 and Ntn1 during Hair Cell Protection in the Neonatal Murine Cochlea. Neurosci. Lett. 2014, 560, 7–11. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, H.; Zhang, P.; Li, H.; Liu, H.; Li, Z. Activation of ERK1/2 and PI3K/Akt by IGF-1 on GAP-43 Expression in DRG Neurons with Excitotoxicity Induced by Glutamate in Vitro. Cell Mol. Neurobiol. 2012, 32, 191–200. [Google Scholar] [CrossRef]
- Kraus, K.S.; Ding, D.; Jiang, H.; Kermany, M.H.; Mitra, S.; Salvi, R.J. Up-Regulation of GAP-43 in the Chinchilla Ventral Cochlear Nucleus after Carboplatin-Induced Hearing Loss: Correlations with Inner Hair Cell Loss and Outer Hair Cell Loss. Hear. Res. 2013, 302, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Kurioka, T.; Matsunobu, T.; Satoh, Y.; Niwa, K.; Endo, S.; Fujioka, M.; Shiotani, A. ERK2 Mediates Inner Hair Cell Survival and Decreases Susceptibility to Noise-Induced Hearing Loss. Sci. Rep. 2015, 5, 16839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Yuan, H.; Talaska, A.E.; Hill, K.; Sha, S.-H. Increased Sensitivity to Noise-Induced Hearing Loss by Blockade of Endogenous PI3K/Akt Signaling. J. Assoc. Res. Otolaryngol. 2015, 16, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ruel, J.; Ladrech, S.; Bonny, C.; van de Water, T.R.; Puel, J.-L. Inhibition of the C-Jun N-Terminal Kinase-Mediated Mitochondrial Cell Death Pathway Restores Auditory Function in Sound-Exposed Animals. Mol. Pharmacol. 2007, 71, 654–666. [Google Scholar] [CrossRef] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C.-Y. Cellular Mechanisms of Noise-Induced Hearing Loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.J.; Thorne, P.R.; Vlajkovic, S.M. Noise-Induced Cochlear Inflammation. World J. Otorhinolaryngol. 2013, 3, 89–99. [Google Scholar] [CrossRef]
- Maulucci, G.; Troiani, D.; Eramo, S.L.M.; Paciello, F.; Podda, M.V.; Paludetti, G.; Papi, M.; Maiorana, A.; Palmieri, V.; De Spirito, M.; et al. Time Evolution of Noise Induced Oxidation in Outer Hair Cells: Role of NAD(P)H and Plasma Membrane Fluidity. Biochim. Biophys. Acta 2014, 1840, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Nakagawa, T.; Kita, T.; Kada, S.; Yoshimoto, M.; Nakahata, T.; Ito, J. Bone Marrow-Derived Cells Expressing Iba1 Are Constitutively Present as Resident Tissue Macrophages in the Mouse Cochlea. J. Neurosci. Res. 2008, 86, 1758–1767. [Google Scholar] [CrossRef]
- Hirose, K.; Discolo, C.M.; Keasler, J.R.; Ransohoff, R. Mononuclear Phagocytes Migrate into the Murine Cochlea after Acoustic Trauma. J. Comp. Neurol. 2005, 489, 180–194. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Fujioka, M.; Kanzaki, S.; Okano, H.J.; Shibata, S.; Yamashita, D.; Masuda, M.; Mihara, M.; Ohsugi, Y.; Ogawa, K.; et al. Blockade of Interleukin-6 Signaling Suppressed Cochlear Inflammatory Response and Improved Hearing Impairment in Noise-Damaged Mice Cochlea. Neurosci. Res. 2010, 66, 345–352. [Google Scholar] [CrossRef]
- Cai, Q.; Vethanayagam, R.R.; Yang, S.; Bard, J.; Jamison, J.; Cartwright, D.; Dong, Y.; Hu, B.H. Molecular Profile of Cochlear Immunity in the Resident Cells of the Organ of Corti. J. Neuroinflamm. 2014, 11, 173. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, M.; Kanzaki, S.; Okano, H.J.; Masuda, M.; Ogawa, K.; Okano, H. Proinflammatory Cytokines Expression in Noise-Induced Damaged Cochlea. J. Neurosci. Res. 2006, 83, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Kermany, M.H.; Cai, Q.; Cai, C.; Zhou, Y.; Nair, U.; Liu, W.; Yoo, T.J. Experimental Autoimmune Hearing Loss Is Exacerbated in IL-10-Deficient Mice and Reversed by IL-10 Gene Transfer. Gene. Ther. 2012, 19, 228–235. [Google Scholar] [CrossRef]
- Bake, S.; Selvamani, A.; Cherry, J.; Sohrabji, F. Blood Brain Barrier and Neuroinflammation Are Critical Targets of IGF-1-Mediated Neuroprotection in Stroke for Middle-Aged Female Rats. PLoS ONE 2014, 9, e91427. [Google Scholar] [CrossRef]
- Toth, P.; Tucsek, Z.; Tarantini, S.; Sosnowska, D.; Gautam, T.; Mitschelen, M.; Koller, A.; Sonntag, W.E.; Csiszar, A.; Ungvari, Z. IGF-1 Deficiency Impairs Cerebral Myogenic Autoregulation in Hypertensive Mice. J. Cereb. Blood Flow Metab. 2014, 34, 1887–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celaya, A.M.; Rodríguez-de la Rosa, L.; Bermúdez-Muñoz, J.M.; Zubeldia, J.M.; Romá-Mateo, C.; Avendaño, C.; Pallardó, F.V.; Varela-Nieto, I. IGF-1 Haploinsufficiency Causes Age-Related Chronic Cochlear Inflammation and Increases Noise-Induced Hearing Loss. Cells 2021, 10, 1686. https://doi.org/10.3390/cells10071686
Celaya AM, Rodríguez-de la Rosa L, Bermúdez-Muñoz JM, Zubeldia JM, Romá-Mateo C, Avendaño C, Pallardó FV, Varela-Nieto I. IGF-1 Haploinsufficiency Causes Age-Related Chronic Cochlear Inflammation and Increases Noise-Induced Hearing Loss. Cells. 2021; 10(7):1686. https://doi.org/10.3390/cells10071686
Chicago/Turabian StyleCelaya, Adelaida M., Lourdes Rodríguez-de la Rosa, Jose M. Bermúdez-Muñoz, José M. Zubeldia, Carlos Romá-Mateo, Carlos Avendaño, Federico V. Pallardó, and Isabel Varela-Nieto. 2021. "IGF-1 Haploinsufficiency Causes Age-Related Chronic Cochlear Inflammation and Increases Noise-Induced Hearing Loss" Cells 10, no. 7: 1686. https://doi.org/10.3390/cells10071686
APA StyleCelaya, A. M., Rodríguez-de la Rosa, L., Bermúdez-Muñoz, J. M., Zubeldia, J. M., Romá-Mateo, C., Avendaño, C., Pallardó, F. V., & Varela-Nieto, I. (2021). IGF-1 Haploinsufficiency Causes Age-Related Chronic Cochlear Inflammation and Increases Noise-Induced Hearing Loss. Cells, 10(7), 1686. https://doi.org/10.3390/cells10071686