Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine
Abstract
:1. Introduction into Human Erythropoietin (EPO): Brief History and Structure
2. Homeostatic Regulation of EPO Production via an Oxygen-Sensitive Feedback Loop
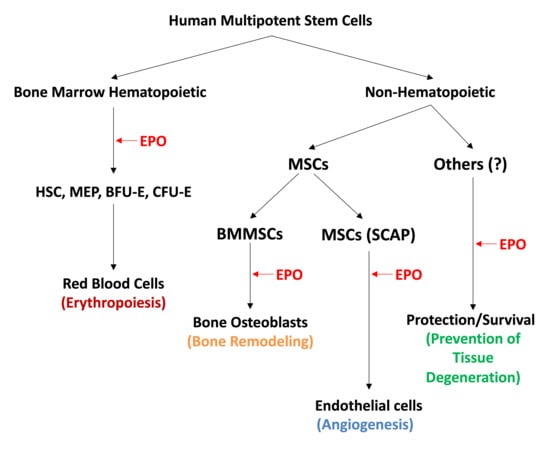
| 1. Bone marrow erythropoiesis via stimulation of proliferation, survival and differentiation of erythroid progenitors into mature red blood cells (RBCs) [3,4,5,18,21] |
| 2. Bone remodeling by activation of the osteoblast bone-forming activity [22,23,24,25,26,27] |
| 3. Modulation of innate and adaptive immunity and inhibition of inflammation [28,29,30,31] |
| 4. Angiogenesis via endothelial transdifferentiation of mesenchymal stem cells (MSCs) [27,32,33,34,35,36,37,38,39] |
| 5. Wound-healing from skeletal muscle injuries via differentiation of muscle progenitors and MSCs [40,41,42,43,44,45] |
| 6. Protection from heart ischemia by increasing the survival of heart cells and endothelial transdifferentiation (angiogenesis) of MSCs [32,41,46,47,48,49,50] |
| 7. Brain protection from ischemic injuries and degenerative disorders via protection of neurons [14,16,51,52,53,54] |
| 8. Inhibition of adipogenesis, fat accumulation and obesity [26] |
| 9. Regulation of energy metabolism via the activation of mitochondrial bioenergetics [21,26,55,56] |
3. EPO Binds to EPO-R in BM Hematopoietic Progenitors and Promotes Erythroid Differentiation
4. EPO Promotes Bone Remodeling
5. EPO Induces Angiogenic Differentiation of Human Endothelial Cells (ECs) and Mesenchymal Stem Cells (MSCs)
6. EPO: From Tissue Protection to Modulation of the Innate and Adaptive Immune Responses
7. EPO Biosimilars
8. Implications of EPO-Induced Differentiation of HSCs and Multipotent MSCs in Regenerative Medicine
9. Conclusions and Future Challenges
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bunn, H.F. Erythropoietin. Cold Spring Harb. Perspect. Med. 2013, 3, ao111619. [Google Scholar] [CrossRef] [Green Version]
- Jelkman, W. Physiology and Pharmacology of Erythropoietin. Transfus. Med. Hemother. 2013, 40, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Broxmeyer, H.E. Erythropoietin: Multiple targets, actions and modifying influences for biological and clinical considerations. J. Exp. Med. 2013, 210, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Tsiftsoglou, A.S.; Vizirianakis, I.S.; Strouboulis, J. Erythropoiesis: Model systems, molecular regulation and developmental processes. IUBMB Life 2009, 61, 800–830. [Google Scholar] [CrossRef]
- Bhoopalan, S.V.; Jun-Huang, L.; Weiss, M.J. Erythropoietin regulation of red blood cell production: From bench to bedside and back. F1000Research 2020, 9, F1000 Faculty Rev-1153. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; de Castro, L.F.; Robey, P.G.; Noguchi, C.T. Erythropoietin modulates bone marrow stromal cell differentiation. Bone Res. 2019, 7. [Google Scholar] [CrossRef]
- McDonald, J.; Lin, F.K.; Goldweisser, E. Cloning, sequencing and evolutionary analysis of the mouse erythropoietin gene. Mol. Cell. Biol. 1986, 6, 842–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.K.; Suggs, S.; Lin, C.H.; Browne, J.K.; Smalling, R. Cloning and expression of the human erythropoietin gene. Proc. Natl. Acad. Sci. USA 1985, 82, 7580–7584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisele, A.S.; Cosgrove, J.; Magniez, A.; Tubenf, E.; Beuto, T.; Cayrac, E.; Tak, T.; Lyne, A.M.; Urbanus, J.; Perie, L. Erythropoietin directly affects single hematopoietic stem cell differentiation after transplantation. BioRxiv 2020. [Google Scholar] [CrossRef]
- Enlev, A. Humoral regulation of red cell production. Blood 1953, 8, 349–357. [Google Scholar] [CrossRef]
- Jacobson, L.O.; Goldwasser, E.; Fried, N. Role of kidney in erythropoiesis. Nature 1957, 179, 633–634. [Google Scholar] [CrossRef]
- Miyake, T.; Klug, K.; Goldwasser, E. Purification of human erythropoietin. J. Biol. Chem. 1977, 252, 5558–5564. [Google Scholar] [CrossRef]
- Goldwasser, E.; Kung, K.H. Purification of erythropoietin. Proc. Natl. Acad. Sci. USA 1971, 68, 697–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, A.D.; Lodish, H.F.; Wong, G.G. Expression cloning of the mouse erythropoietin receptor. Cell 1989, 57, 277–285. [Google Scholar] [CrossRef]
- Rey, F.; Balsari, A.; Giallongo, T.; Ottoherghi, S.; De Giulio, A.M.; Samaja, M.; Carelli, S. Erythropoietin as a neuroprotective molecule: An overview of its therapeutic potential in neurodegenerative diseases. Am. Soc. Neurochem. 2019, 11, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, R. Pleiotropic functions of Erythropoietin. Intern. Med. 2003, 42, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Kietzmann, T. Hypoxia inducible erythropoietin expression: Details matter. Hematologica 2020, 105, 2704–2706. [Google Scholar] [CrossRef] [PubMed]
- Palis, J.; Koniski, A. Functional analysis of erythroid progenitors by colony-forming assays. Methods Mol. Biol. 2018, 1698, 117–132. [Google Scholar]
- Watts, D.; Gaete, S.; Rodriguez, D.; Hoojewijs, D.; Rauner, M.; Sormendi, S.; Wielockx, B. Hypoxia pathway proteins are master regulators of erythropoiesis. Int. J. Mol. Sci. 2020, 21, 8131. [Google Scholar] [CrossRef]
- The Nobel Assembly at Karolinska Institutet. Press Release: The Nobel Prize in Physiology or Medicine 2019; Jointly to Kaelin, W.G.; Jr., Ratcliffe, P.J. and Semenza, C.l. for their discoveries of how cells sense and adapt to oxygen availability; The Nobel Prize: Stockholm, Sweden, 2019. [Google Scholar]
- Wang, L.; Di, L.; Noguchi, C.T. Erythropoietin: A novel versatile player regulating Energy Metabolism and beyond the erythroid system. Int. J. Biol. Sci. 2014, 10, 921–999. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Spencer, J.A.; Koh, B.I.; Kobayashi, T.; Fujisaki, I.; Lin, C.P.; Kroneberg, H.M.; Scadden, D. Endogenous BMMSCs are dynamic, fate restricted participants in bone maintenance and regeneration. Cell Stem Cell 2012, 10, 259–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, P.; Rimunnicci, M.; Gronthos, S.; Robey, P.G. Bone marrow stromal stem cells: Nature, biology and potential applications. Stem Cells 2001, 19, 180–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, P. Stem cells and Bone. A historical perspective. Bone 2015, 70, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wang, X.; Niyibizi, C. Bone marrow stromal cells contribute to bone formation following infusion into femoral cavities of a mouse model of osteogenesis imperfecta. Bone 2010, 47, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Suresh, S.; Lee, J.; Noguchi, C.T. Effects of erythropoietin in white adipose tissue and bone microenvironment. Front. Cell Dev. Biol. 2020, 8, 584696. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, F.; He, Q.; Tsang, W.P.; Lu, L. EPO promotes bone repair through enhanced cartilaginous callus and angiogenesis. PLoS ONE 2014, 9, e102010. [Google Scholar] [CrossRef] [Green Version]
- Cantarelli, C.; Angelleti, A.; Cravedi, P. Erythropoietin, a multifaceted protein with innate and adaptive modulatory activity. Am. J. Transplant. 2019, 19, 2407–2414. [Google Scholar] [CrossRef]
- Peng, B.; Kong, G.; Yang, C.; Ming, Y. Erythropoietin and its derivatives: From tissue protection to immune regulation. Cell Death Dis. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Maeda, Y.; Li, W.; Lu, W.; Cook, S.; Dowing, P. Erythropoietin: A potent inducer of peripheral immune/inflammatory modulation in autoimmune EAE. PLoS ONE 2008, 3, e1924. [Google Scholar] [CrossRef] [Green Version]
- Regmi, S.; Pathak, S.; Kim, J.O.; Yong, C.S.; Jeong, J.H. Mesenchymal stem cell therapy for the treatment of inflammatory disease: Challenges, opportunities and future perspectives. Eur. J. Cell Biol. 2019, 98, 151041. [Google Scholar] [CrossRef] [PubMed]
- Kimakova, P.; Solar, P.; Solarova, Z.; Komel, R.; Debeljak, N. Erythropoietin and its angiogenic activity. Int. J. Mol. Sci. 2017, 18, 1519. [Google Scholar] [CrossRef]
- Carlini, R.G.; Reyes, A.A.; Rothstein, M. Recombinant human erythropoietin stimulates angiogenesis in vitro. Kidney Int. 1995, 47, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Anagnostou, A.; Liu, Z.; Steiner, M.; Chin, K.; Lee, E.S.; Kessimian, N.; Noguchi, C.T. Erythropoietin receptor mRNA expression in human endothelial cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3974–3978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribatti, D.; Presta, M.; Vacca, A.; Ria, R.; Guiliani, R.; Dell’Era, P.; Nico, B.; Roncali, L.; Dammacco, F. Human erythropoietin induces a proangiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood 1999, 93, 2627–2636. [Google Scholar] [CrossRef]
- Holstein, J.H.; Orth, M.; Scheuer, C.; Tammi, A.; Becker, S.C.; Garcia, P.; Histing, T.; Morsdorf, P.; Klein, M.; Pohlemann, T.; et al. Erythropoietin stimulates bone formation, cell proliferation and angiogenesis in a femoral segmented defect model in mice. Bone 2011, 49, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Oswald, J.; Boxberger, S.; Jørgensen, B.; Feldmann, S.; Ehninger, G.; Bornhäuser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Kritis, A.; Andeadis, D.; Papachistou, E.; Leyhausen, G.; Koidis, P.; Geutsen, W.; Tsiftsoglou, A. Angiogenic potential and Secretome of human Apical Papilla Mesenchymal stem cells in Various Stress microenvironment. Stem Cells Dev. 2015, 24, 2496–2512. [Google Scholar] [CrossRef] [Green Version]
- Koutsoumparis, A.; Vassili, A.; Bakopoulou, A.; Ziouta, A.; Tsiftsoglou, A.S. Erythropoietin (rhEPOa) promotes endothelial transdifferentiation of stem cells of the apical papilla. Arch. Oral Biol. 2018, 96, 96–103. [Google Scholar] [CrossRef]
- Rundqvist, H.; Rullman, E.; Sundberg, C.J.; Fischer, H.; Eisleitner, K.; Stahlberg, M. Activation of the erythropoietin receptor in human skeletal muscle. Eur. J. Endocrinol. 2009, 161, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Brines, M.; Grasso, G.; Fiorlaliso, F.; Sfacteria, A.; Ghezzi, P.; Fratelli, M.; Latini, R.; Xie, O.W.; Smart, J.; Su-Rick, C.-J.; et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc. Natl. Acad. Sci. USA 2004, 101, 14907–14912. [Google Scholar] [CrossRef] [Green Version]
- Pledge, U.; Belhage, B.; Guadalupe-Grau, A.; Andersen, P.R.; Lundby, C.; Dela, F. Erythropoietin treatment enhances muscle mitochondrial capacity in humans. Front. Physiol. 2012, 3, 50. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Susuki, N.; Yamamamoto, M.; Gassmann, M.; Noguchi, C.T. Endogenous erythropoietin signaling facilitates skeletal muscle repair and recovery following pharmacologically induced damage. FASEB J. 2012, 23, 2847–2858. [Google Scholar] [CrossRef] [Green Version]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, W.M.; Nosti, L.; Tuan, R.S. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res. Ther. 2012, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Van Der Meer, P.; Lipsic, E.; Henning, R.H.; Van Der Velden, J.; Voors, A.A. Erythropoietin induces neovascularization and improves cardiac function in rats with heart failure after myocardial infraction. J. Am. Coll. Cardiol. 2005, 46, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Miura, T.; Ischida, H.; Miki, T.; Tanno, M.; Yano, M.; Sato, T.; Hotta, H.; Shimamoto, K. Limitation of infract size by erythropoietin is associated with translocation of AKT to the mitochondria after reperfusion. Clin. Exp. Pharmacol. 2008, 135, 799–808. [Google Scholar]
- Calvinio, L.; Latini, R.; Kajsgera, J.; Leri, A.; Anversa, P.; Ghezzi, P.; Salio, M.; Cerami, A.; Brines, M. Recombinant human erythropoietin protects the myocardium-reperfusion injury and promotes beneficial remodeling. Proc. Natl. Acad. Sci. USA 2003, 100, 4802–4806. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Manalo, D.J.; Wei, G.; Rodriguez, R.; Fox-Tablot, K.; Lu, H.; Zweiter, J.L.; Semenza, G.L. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia reperfusion injury. Circulation 2003, 108, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Parsa, C.J.; Matsumoto, A.; Kim, J.; Riel, R.U.; Pascal, L.S.; Walton, G.B. A novel protective effect of erythropoietin in the infracted heart. J. Clin. Investig. 2003, 112, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, M.; Wen, T.C.; Matsuda, S.; Masuda, S.; Morishita, E.; Nagao, M. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc. Natl. Acad. Sci. USA 1998, 95, 4635–4640. [Google Scholar] [CrossRef] [Green Version]
- Tsai, P.T.; Ohab, J.J.; Kertesz, N.; Groszer, M.; Matter, C.; Gao, J.; Liu, X.; Wu, H.; Carmichael, S.T. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J. Neurosci. 2006, 26, 1269–1274. [Google Scholar] [CrossRef] [Green Version]
- Allers, C.; Jones, J.A.; Lasala, G.F.; Minguell, J.J. Mesenchymal stem therapy for the treatment of amyotrophic lateral sclerosis: Signals for hope. Regen. Med. 2014, 9, 637–697. [Google Scholar] [CrossRef]
- Schneider, F.; Horowitz, A.; Lesch, K.D.; Dandekar, T. Delaying memory decline: Different options and emergency solutions. Transl. Psychiatry 2020, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Suresh, S.; Rajvanshi, P.K.; Noguchi, C.T. The many facets of erythropoietin Physiologic and Metabolic Response. Front. Physiol. 2020, 10, 1534. [Google Scholar] [CrossRef] [Green Version]
- Wang, I.; Jia, Y.; Rogers, H.; Susuki, N.; Gassmann, M.; Wang, Q. Erythropoietin contributes to slow oxidative muscle fiber specification via PGC-alpha and AMPK activation. Int. J. Biochem. Cell Biol. 2013, 45, 1155–1164. [Google Scholar] [CrossRef] [Green Version]
- Jelkmann, W. Regulation of erythropoietin production. J. Physiol. 2011, 589, 1251–1258. [Google Scholar] [CrossRef]
- Liu, C.; Shen, K.; Lin, Z.; Noguchi, C.T. Regulated human erythropoietin receptor expression in mouse brain. J. Chem. Biol. 1977, 272, 32395–32400. [Google Scholar] [CrossRef] [Green Version]
- Bakopoulou, A.; Leyhansen, C.; Volic, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteogenic/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Procop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Muraglia, A.; Cancedda, R.; Quarto, R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hieraxhical model. J. Cell Sci. 2000, 113, 1161–1166. [Google Scholar] [CrossRef]
- Jiang, Y.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.D.; Ortiz-Gonzalez, X.R.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M.; et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002, 418, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Ucelli, A.; Moretta, P.; Pistoia, V. Mesenchymal stromal cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Domenici, M.; Le Blanc, K.; Mueller, I.; Contenbach, S.; Marini, F.; Krause, S.D. Minimal criteria defining multipotent mesenchymal stromal cells. The International Society for Cell Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Nombela-Arrieta, C.; Ritz, J.; Silberstein, L.E. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishan, A.; Storb, T.; Pillai, M.J. Primary marrow derived stromal cells: Isolation and manipulation. Methods Mol. Biol. 2013, 1035, 75–101. [Google Scholar]
- Suresh, S.; Lee, J.; Noguchi, C.T. Erythropoietin signaling in osteoblasts is required for normal bone formation and the bone loss during erythropoietin –stimulated erythropoiesis. FASEB 2020, 34, 11685–11697. [Google Scholar] [CrossRef]
- Anagnostou, A.; Lee, E.S.; Kessimian, N.; Levinson, R.; Steiner, M. Erythropoietin has a mitogenic and positive chemotactic effect on endothial cells. Proc. Natl. Acad. Sci. USA 1990, 87, 5978–5982. [Google Scholar] [CrossRef] [Green Version]
- Heeschen, C.; Aicher, A.; Lehmann, R.; Fitchtscherer, S.; Vasa, M.; Urbich, C.; Mildner-Rihm, C.; Martin, H.; Zeiber, A.M.; Dimmeler, S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 2003, 102, 1340–1346. [Google Scholar] [CrossRef] [Green Version]
- Beleshin–Cokic, B.B.; Cokic, V.P.; Yu, X.; Weksler, B.B.; Schechter, A.N.; Noguchi, C.T. Erythropoietin and hypoxia stimulate erythropoietin-receptor and nitric oxide production by endothelial cells. Blood 2004, 104, 2073–2080. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.; Hodgson-Garms, M.; Fritch, J.E.; Genever, P. Multiplicity of mesenchymal stromal cells: Finding the right route to therapy. Front. Immunol. 2019, 10, 1112. [Google Scholar] [CrossRef] [Green Version]
- Friedenstein, A.J.; Chailakhjan, R.K.; Ladykina, K.S. The development of fibroblastic colonies in monolayer cultures of guinea pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar]
- Bianco, P.; Robey, P.G.; Saggio, I.; Riminucci, M. Mesenchymal stem cells in human bone marrow (skeletal stem cells): A critical discussion of their nature, identity and significance in incurable skeletal disease. Hum. Gene Ther. 2020, 21, 1057–1066. [Google Scholar] [CrossRef] [Green Version]
- Somoza, R.R.; Corren, D.; Caplan, A.I. Role of mesenchymal stem cells as medicinal sighting cells Nature Protocols (poster).
- Lv, F.V.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. The surface markers and identity of Human Mesenchymal Stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Monterubbianesi, R.; Bencum, M.; Pagella, P.; Woloszyl, P.A.; Orsiki, G.; Mitsiadis, J.A. Comparative in vitro study of the osteogenic and adipogenic potential of human dental pulp stem cells, gingival fibroblasts and foreskin fibroblasts. Sci. Rep. 2019, 9, 1761. [Google Scholar] [CrossRef]
- Arthur, A.; Rychkov, G.; Shl, S.; Kobbar, S.A.; Gronthos, S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef] [Green Version]
- Brines, M.; Cerami, A. Erythropoietin mediated tissue protection reducing collateral damage from the primary injury response. J. Intern. Med. 2008, 264, 405–432. [Google Scholar] [CrossRef]
- Coleman, T.; Brines, M. Science review: Recombinant human erythropoietin in critical illness: A role beyond anemia? Crit. Care 2004, 8, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, D.; Susuma, K.; Matsui, S.; Kurimoto, M.; Kita, M.; Suzuma, I.; Ohashi, H.; Objima, T.; Murakami, T. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N. Engl. J. Med. 2005, 353, 782–792. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Zhou, X.j.; Liaso, S.Y. Erythropoietin enhances recovery from cis-platinum–induced acute renal failure. Am. J. Physiol. 1994, 266, F360–F366. [Google Scholar]
- Cassis, P.; Galon, L.; Benigini, A.; Mister, M.; Pezzota, A.; Solini, S. Erythropoietin, but not the correction of anemia alone, protects from chronic kidney allograft injury. Kidney Int. 2012, 81, 903–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, F.; Kamar, N.; Pellet, N.; Lang, P.; Durrbach, A.; Lebranchu, Y. High dose of epoetin beta in the first weeks following renal transplantation and delayed graft function: Results of the Neo-PDGF study. Am. J. Transpl. 2010, 10, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Krendyukov, A.; Schiesti, M.; Gascon, P. Epoetin biosimilars in the treatment of chemotherapy induced anemia: 10 years’ experience gained. Biodrugs 2018, 32, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garson, P.; Kredyukov, A.; Mathienson, N.; Aapro, M. Epoetin alfa for the treatment of myelodysplastic syndrome related anemia: A review of clinical data, clinical guidelines and treatment protocols. Leuk. Res. 2019, 81, 35–42. [Google Scholar] [CrossRef]
- Brinks, V.; Hawe, A.; Basmeleh, A.H.H.; Joachin-Rodriquez, L.; Haseberg, R.; Somsen, G.W.; Jiskoot, W.; Schellekens, H. Quality of original and Biosimilar Epoetins Products. Pharm. Res. 2011, 28, 386–393. [Google Scholar] [CrossRef] [Green Version]
- Editor Comment. Biosimilars of Epoietin Alpha; Generics and Biosimilar Initiative GaBi: Antwerp, Belgium, 2014. [Google Scholar]
- Minghetti, P.; Rocco, P.; Ciluizo, F.; del Veccio, L.; Locatelli, F. The regulatory framework of biosimilars in the European Union. Drug Discov. Today 2012, 17, 63–70. [Google Scholar] [CrossRef]
- Tsiftsoglou, A.S.; Ruiz, S.; Schneider, C.K. Development and regulation of biosimilars: Current status and future challenges. Biodrugs 2013, 7, 209–211. [Google Scholar] [CrossRef]
- Schneider, C.K.; Borg, J.J.; Ehman, F.; Ekman, N.; Heinonen, E.; Ho, K.; Hoefuagel, M.H.; van der Plass, R.M.; van der Stappen, A.J.; Thorpe, R.; et al. In support of the European Union Biosimilar framework. Nat. Biotechnol. 2012, 30, 745–748. [Google Scholar] [CrossRef] [Green Version]
- Tsiftsoglou, A.S.; Trouvin, J.H.; Calvo, G.; Ruiz, S. Demonstration of biosimilarity, extrapolation of indications and other challenges related to Biosimilars in Europe. Biodrugs 2014, 28, 479–486. [Google Scholar] [CrossRef]
- Ogilvie, M.; Yu, X.; Nicolas-Metral, V.; Pulido, S.M.; Li, C.; Ruegg, U.T. Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J. Biol. Chem. 2000, 275, 39754–39761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J. The meaning, the sense and the significance translating the science of mesenchymal stem cells into medicine. Nat. Med. 1999, 107, 725–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Oiao, Y.M.; Liu, Y.G.; Liu, D.; Hu, J.M.; Liao, J.; Li, M.; Guo, Y. Bone marrow derived mesenchymal stem cells pretreated with erythropoietin accelerates the repair of acute kidney injury. Cell Biosci. 2020, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, X.; He, J.; Zhao, W. Influence of erythropoietin on microvesicles derived from mesenchymal stem cells protecting renal function of chronic kidney disease. Stem Cell Res. Ther. 2015, 6, 100. [Google Scholar] [CrossRef] [Green Version]
- Perucca, S.; Di Palma, A.; Piccaluga, P.P.; Gemelli, C.; Zoratti, E.; Bassi, G.; Giacopuzzi, E.; Lojacono, A.; Borsani, G.; Tagliafico, E.; et al. Mesenchymal stromal cells (MSCs) induce ex vivo proliferation and erythroid commitment of cord blood hematopoietic stem cells (CB-CD34+ cells). PLoS ONE 2017, 12, e0172430. [Google Scholar] [CrossRef] [Green Version]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Yuan, D.; Wong, P.; Zhu, L.; Dissanayaka, W.I.; Green, D.W.; Tong, E.H.; Jim, L.; Zang, C. Coculture of stem cells from apical papilla and human umbilical vein endothelial cell under hypoxia increases the formation of three-dimensional vessel like-structures in vitro. Tissue Eng. Part A 2015, 21, 11631172. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.W.; Shin, Y.Y.; Seo, Y.; Kim, H.S. Therapeutic functions of stem cells from oral cavity: An update. Intern. J. Mol. Sci. 2020, 21, 4389. [Google Scholar] [CrossRef]
- Ouchi, T.; Nakagama, T. Mesenchymal stem cell-based tissue regeneration therapies for periodontitis. Regen. Ther. 2020, 14, 72–78. [Google Scholar] [CrossRef]
- EL Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; Dorfer, C.E.; Fanzy, E.L.; Sayed, K.M. Dental stem cell derived secretome conditioned medium: The future for regenerative therapeutics applications. Stem Cell Int. 2020, 2020, 7593402. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.C.; Narayannan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools from stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials 2016, 111, 103–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillkens, P.; Bronckaers, A.; Ratajczak, J.; Gervois, P.; Wolfs, E.; Lambrichts, I. The angiogenic potential of DPScs and SCSPs in an in vivo Model of Dental Pulp Regeneration. Stem Cell Int. 2017, 2017, 2582080. [Google Scholar]
- Hillkens, N.; Meschi, P.; Lambrechts, P.; Bronckaers, A.; Lambrichts, I. Dental stem Cells in pulp regeneration: Near future or long road ahead. Stem Cells Dev. 2015, 34, 1610–1622. [Google Scholar] [CrossRef]
- Schneider, C.K.; Salmikangas, P.; Jilma, B.; Flamion, B.; Todorova, L.P.; Paphitou, A.; Haunerova, I.; Maimets, T.; Trouvin, J.H.; Flory, E.; et al. Challenges with Advanced Therapy Medicinal products and how to meet them. Nat. Rev. Drug Discov. 2010, 9, 195–201. [Google Scholar]
- Detela, G.; Lodge, A. EU Regulatory Pathways for ATMPs: Standard, Accelerated and adaptive Pathways for Marketing Authorization. Mol. Ther. Methods Clin. Dev. 2019, 13, 205–231. [Google Scholar] [CrossRef] [Green Version]
- Salmikangas, P.; Menezes-Ferreira, M.; Reisch, I.; Tsiftsoglou, A.; Kyselovic, J. Manufacturing, characterization and control of cell based medicinal products: Challenges paradigms toward commercial use. Regen. Med. 2015, 10, 65–78. [Google Scholar] [CrossRef]


| Tissue of Origin | Induced to Differentiate into |
|---|---|
| Bone marrow (BM) | Osteoblast, osteoclasts, stromal cells, pericytes |
| Umbilical cord blood (UCB) | Adipocytes, chondrocytes, osteoblasts, epithelial cells |
| Brain | Neuron-like cells (?) |
| Heart | Cardiomyocytes |
| Liver | Hepatocytes |
| Oral cavity (DPSCs, SCAP) | Osteoblast/odontoblast, endothelial cells, neuron-like cells (a) |
| Kidney | Interstitial tubular cells |
| Fat | Chondrocytes, skeletal muscle cells (myoblasts) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiftsoglou, A.S. Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells 2021, 10, 2140. https://doi.org/10.3390/cells10082140
Tsiftsoglou AS. Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells. 2021; 10(8):2140. https://doi.org/10.3390/cells10082140
Chicago/Turabian StyleTsiftsoglou, Asterios S. 2021. "Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine" Cells 10, no. 8: 2140. https://doi.org/10.3390/cells10082140
APA StyleTsiftsoglou, A. S. (2021). Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells, 10(8), 2140. https://doi.org/10.3390/cells10082140