Gut Leakage Markers in Response to Strenuous Exercise in Patients with Suspected Coronary Artery Disease
Abstract
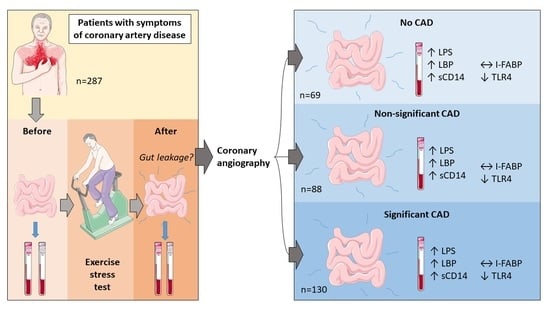
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exercise Stress Test
2.3. Laboratory Methods
2.4. Coronary Angiography
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Exercise Performance
3.3. Gut Leakage Markers at Baseline
3.4. Gut Leakage Markers and Strenuous Exercise
4. Discussion
4.1. Effect of Exercise on Gut Leakage Markers
4.2. Difference between Groups of CAD
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franklin, B.A.; Thompson, P.D.; Al-Zaiti, S.S.; Albert, C.M.; Hivert, M.F.; Levine, B.D.; Lobelo, F.; Madan, K.; Sharrief, A.Z.; Eijsvogels, T.M.; et al. Exercise-Related Acute Cardiovascular Events and Potential Deleterious Adaptations Following Long-Term Exercise Training: Placing the Risks Into Perspective-An Update: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e705–e736. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Corra, U.; Cosyns, B.; Deaton, B.; Graham, I.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [PubMed]
- Palmefors, H.; DuttaRoy, S.; Rundqvist, B.; Börjesson, M. The effect of physical activity or exercise on key biomarkers in atherosclerosis--a systematic review. Atherosclerosis 2014, 235, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.J.; Virtanen, K.A.; LÖyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Gao, R.; Zhang, Y.; Pan, D.; Zhu, Y.; Zhang, X.; Yang, R.; Jialng, R.; Xu, Y.; Qin, H. Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol. Genom. 2018, 50, 893–903. [Google Scholar] [CrossRef]
- Mittleman, M.A.; Maclure, M.; Tofler, G.H.; Sherwood, J.B.; Goldberg, R.J.; Muller, J.E. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N. Engl. J. Med. 1993, 329, 1677–1683. [Google Scholar] [CrossRef]
- Thompson, P.D.; Franklin, B.A.; Balady, G.J.; Blair, S.N.; Corrado, D.; Estes, N.A., 3rd; Fulton, J.E.; Gordon, N.F.; Haskell, W.L.; Link, M.S.; et al. Exercise and acute cardiovascular events placing the risks into perspective: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 2007, 115, 2358–2368. [Google Scholar] [PubMed] [Green Version]
- Bosenberg, A.T.; Brock-Utne, J.G.; Gaffin, S.L.; Wells, M.T.; Blake, G.T. Strenuous exercise causes systemic endotoxemia. J. Appl. Physiol. 1988, 65, 106–108. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.H.; Senden, J.; Saris, W.H.; Wagenmakers, A.J.M. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef]
- Zuhl, M.; Schneider, S.; Lanphere, K.; Conn, C.; Dokladny, K.; Moseley, P. Exercise regulation of intestinal tight junction proteins. Br. J. Sports Med. 2014, 48, 980–986. [Google Scholar] [CrossRef] [PubMed]
- van Wijck, K.; Lenaerts, K.; Grootjans, J.; Wijnands, K.A.; Poeze, M.; van Loon, L.J.; DeJong, C.H.C.; Buurman, W.A. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: Strategies for evaluation and prevention. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G155–G168. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef] [Green Version]
- Cwikiel, J.; Seljeflot, I.; Fagerland, M.W.; Wachtell, K.; Arnesen, H.; Berge, E.; Flaa, A. High-sensitive cardiac Troponin T and exercise stress test for evaluation of angiographically significant coronary disease. Int. J. Cardiol. 2019, 287, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Morise, A.P.; Haddad, W.J.; Beckner, D. Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am. J. Med. 1997, 102, 350–356. [Google Scholar] [CrossRef]
- Borg, G.A. Perceived exertion. Exerc. Sport Sci. Rev. 1974, 2, 131–153. [Google Scholar] [CrossRef]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cwikiel, J.; Seljeflot, I.; Berge, E.; Njerve, I.U.; Ulsaker, H.; Arnesen, H.; Flaa, A. Effect of strenuous exercise on mediators of inflammation in patients with coronary artery disease. Cytokine 2018, 105, 17–22. [Google Scholar] [CrossRef]
- Oliveira, M.; Gleeson, M. The influence of prolonged cycling on monocyte Toll-like receptor 2 and 4 expression in healthy men. Eur. J. Appl. Physiol. 2010, 109, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Slusher, A.L.; Zúñiga, T.M.; Acevedo, E.O. Maximal Exercise Alters the Inflammatory Phenotype and Response of Mononuclear Cells. Med. Sci. Sports Exerc. 2018, 50, 675–683. [Google Scholar] [CrossRef]
- Zarember, K.A.; Godowski, P.J. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol. 2002, 168, 554–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri, B.; Sohrabi, B.; Movassaghpour, A.A.; Mashayekhi, S.; Garjani, A.; Shokri, M.; Pezeshkian, M.; Garjani, A. Hydrocortisone reduces Toll-like receptor 4 expression on peripheral CD14+ monocytes in patients undergoing percutaneous coronary intervention. Iran Biomed. J. 2014, 18, 76–81. [Google Scholar]
- Van Wijck, K.; Lenaerts, K.; van Loon, L.J.; Peters, W.H.; Buurman, W.A.; Dejong, C.H. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS ONE 2011, 6, e22366. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.P.B.; Texeira, T.F.S.; Ferreira, A.B.; Peluzio, M.D.C.G.; Alfenas, R.D.C.G. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Et Biophys. Acta 2009, 1788, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.K.; Hankey, J.; Wright, A.; Marczak, S.; Hemming, K.; Allerton, D.M.; Ansley-Robson, P.; Costa, R.J.S. The Impact of a 24-h Ultra-Marathon on Circulatory Endotoxin and Cytokine Profile. Int. J. Sports Med. 2015, 36, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, M.A.; Leggate, M.; Viana, J.L.; King, J.A. The effect of physical activity on mediators of inflammation. Diabetes Obes. Metab. 2013, 15 (Suppl. 3), 51–60. [Google Scholar] [CrossRef]
- Aune, S.K.; Byrkjeland, R.; Solheim, S.; Arnesen, H.; Trøseid, M.; Awoyemi, A.; Seljeflot, I.; Helseth, R. Gut related inflammation and cardiorespiratory fitness in patients with CAD and type 2 diabetes: A sub-study of a randomized controlled trial on exercise training. Diabetol. Metab. Syndr. 2021, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Andersen, G.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| All (n = 287) | No CAD (n = 69) | Non-Significant CAD (n = 88) | Significant CAD (n = 130) | p-Value 1 | p-Value 2 | p-Value 3 | |
|---|---|---|---|---|---|---|---|
| Age (years) * | 62.4 (40.1–87.2) | 60.8 (43.8–80.6) | 61.7 (40.1–79.6) | 63.7 (40.9–87.2) | 0.079 | 0.085 | 0.037 |
| Sex; female, n (%) | 102 (35.5) | 44 (63.8) | 32 (36.4) | 26 (20.0) | <0.001 | <0.001 | <0.001 |
| BMI (kg/m2) | 26.6 (24.7, 29.6) | 26.8 (24.3, 30.4) | 26.3 (24.7, 29.0) | 26.6 (25.0, 30.0) | 0.271 | 0.954 | 0.524 |
| Diabetes, n (%) | 56 (19.5) | 15 (21.7) | 12 (13.6) | 29 (22.3) | 0.248 | 0.593 | 0.927 |
| Hypertension, n (%) | 169 (58.9) | 34 (49.3) | 50 (56.8) | 85 (65.4) | 0.081 | 0.063 | 0.041 |
| Resting heart rate (bpm) | 68 (61, 77) | 70 (62, 75) | 70 (62, 80) | 67 (59, 75) | 0.076 | 0.630 | 0.189 |
| Hypercholesterolemia, n (%) | 231 (80.5) | 42 (60.9) | 71 (80.7) | 118 (90.8) | <0.001 | <0.001 | <0.001 |
| Smoker, n (%) 4 | 52 (18.1) | 39 (56.5) | 54 (61.4) | 83 (63.9) | 0.602 | 0.348 | 0.391 |
| HbA1c % | 5.7 (5.4, 6.2) | 5.7 (5.4, 6.2) | 5.6 (5.4, 5.9) | 5.7 (5.4, 6.3) | 0.232 | 0.720 | 0.753 |
| Creatinine (μmol/L) | 79 (69, 89) | 73.5 (66, 80.5) | 78 (68, 88) | 82 (73, 93) | <0.001 | <0.001 | <0.001 |
| Total cholesterol (mmol/L) | 4.6 (3.9, 5.6) | 5.3 (4.5, 6.2) | 4.7 (3.9, 5.8) | 4.3 (3.7, 5) | <0.001 | <0.001 | <0.001 |
| LDL (mmol/L) | 2.6 (2.1, 3.5) | 3.0 (2.4, 4.0) | 2.6 (2.0, 3.6) | 2.4 (2.0, 3.0) | 0.001 | 0.002 | <0.001 |
| Medication, n (%) | |||||||
| Platelet inhibitor 5 | 209 (72.8) | 29 (42.0) | 63 (71.6) | 117 (90.0) | <0.001 | <0.001 | <0.001 |
| ACEI/ARB | 103 (35.9) | 17 (24.6) | 35 (39.8) | 51 (39.2) | 0.083 | 0.026 | 0.054 |
| Betablocker | 119 (41.5) | 14 (20.3) | 34 (38.6) | 71 (54.6) | <0.001 | <0.001 | <0.001 |
| Calcium blocker | 47 (16.3) | 5 (7.3) | 14 (15.9) | 28 (21.5) | 0.035 | 0.019 | 0.013 |
| Statins | 203 (70.7) | 34 (49.3) | 60 (68.2) | 109 (83.9) | <0.001 | <0.001 | <0.001 |
| All (n = 287) | No CAD (n = 68) | Non-Significant CAD (n = 88) | Significant CAD (n = 130) | p-Value 1 | p-Value 2 | p-Value 3 | |
|---|---|---|---|---|---|---|---|
| Exercise duration (min:sec) | 09:31 min (07:20, 12:04) | 10:00 min (8:04, 13:24) | 09:54 min (07:24, 11:50) | 09:16 min (06:43, 12:03) | 0.154 | 0.099 | 0.070 |
| METs | 6.7 (5.7, 8.0) | 7.0 (6.1, 8.2) | 7.0 (5.8, 8.0) | 6.3 (5.6, 8.0) | 0.036 | 0.096 | 0.023 |
| Maximal RPE, Borg scale | 17 (15, 18) | 17 (16, 19) | 17 (15, 18) | 17 (15, 17) | 0.010 | 0.037 | 0.006 |
| All (n = 287) | No CAD (n = 69) | Non-Significant CAD (n = 88) | Significant CAD (n = 130) | p-Value 1 | p-Value 2 | p-Value 3 | |
|---|---|---|---|---|---|---|---|
| sCD14 (ng/mL) | 1353 (1217, 1560) | 1419 (1224, 1600) | 1402 (1220, 1607) | 1329 (1194, 1495) | 0.097 | 0.312 | 0.107 |
| LBP (ng/mL) | 15,805 (13,378, 19,045) | 15,033 (12,276, 18,593) | 15,562 (12,723, 19,032) | 16,293 (13,877, 19,233) | 0.415 | 0.318 | 0.232 |
| I-FABP (pg/mL) | 927 (631, 1324) | 943 (677, 1287) | 894 (600, 1341) | 925 (629, 1292) | 0.811 | 0.832 | 0.981 |
| All (n = 101) | No CAD (n = 29) | Non-Significant CAD (n = 27) | Significant CAD (n = 45) | ||||
| LPS (pg/mL) | 77.1 (64.0, 90.2) | 74.3 (60.9, 89.2) | 78.5 (66.3, 94.0) | 78.3 (67.9, 91.2) | 0.661 | 0.372 | 0.441 |
| TLR4 receptor (RQ) | 1.1 (0.9, 1.6) | 1.3 (0.9, 1.7) | 1.1 (0.8, 1.6) | 1.1 (0.9, 1.4) | 0.403 | 0.180 | 0.199 |
| No CAD (n = 69) | Non-Significant CAD (n = 88) | Significant CAD (n = 130) | Δp-Value 1 | Rel Δp-Value 2 | |
|---|---|---|---|---|---|
| sCD14 (ng/mL) | 98 (−2, 192) | 74 (−35, 174) | 82 (−7.5, 180) | p = 0.915 | p = 0.860 |
| LBP (ng/mL) | 954 (471, 1783) | 1188 (419, 1997) | 995 (446, 1966) | p = 0.645 | p = 0.793 |
| I-FABP (pg/mL) | −40 (−188, 146) | −26 (−187, 114) | −29 (−166, 121) | p = 0.914 | p = 0.924 |
| (n = 29) | (n = 27) | (n = 45) | |||
| LPS (pg/mL) | 4.5 (1.4, 17.6) | 4.1 (−2.84, 13.3) | 7.8 (−3.8, 14.3) | p = 0.552 | p = 0.464 |
| TLR4 RQ | −0.3 (−0.70, −0.07) | −0.1 (−0.48, 0.04) | −0.20 (−0.40, 0.02) | p = 0.158 | p = 0.384 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aune, S.K.; Cwikiel, J.; Flaa, A.; Arnesen, H.; Solheim, S.; Awoyemi, A.; Trøseid, M.; Seljeflot, I.; Helseth, R. Gut Leakage Markers in Response to Strenuous Exercise in Patients with Suspected Coronary Artery Disease. Cells 2021, 10, 2193. https://doi.org/10.3390/cells10092193
Aune SK, Cwikiel J, Flaa A, Arnesen H, Solheim S, Awoyemi A, Trøseid M, Seljeflot I, Helseth R. Gut Leakage Markers in Response to Strenuous Exercise in Patients with Suspected Coronary Artery Disease. Cells. 2021; 10(9):2193. https://doi.org/10.3390/cells10092193
Chicago/Turabian StyleAune, Susanne Kristine, Joanna Cwikiel, Arnljot Flaa, Harald Arnesen, Svein Solheim, Ayodeji Awoyemi, Marius Trøseid, Ingebjørg Seljeflot, and Ragnhild Helseth. 2021. "Gut Leakage Markers in Response to Strenuous Exercise in Patients with Suspected Coronary Artery Disease" Cells 10, no. 9: 2193. https://doi.org/10.3390/cells10092193
APA StyleAune, S. K., Cwikiel, J., Flaa, A., Arnesen, H., Solheim, S., Awoyemi, A., Trøseid, M., Seljeflot, I., & Helseth, R. (2021). Gut Leakage Markers in Response to Strenuous Exercise in Patients with Suspected Coronary Artery Disease. Cells, 10(9), 2193. https://doi.org/10.3390/cells10092193