A Novel Technique for the Generation of Substantial Numbers of Functional Resident T Cells from Kidney Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
| Healthy Kidney | Kidney Transplant | |
|---|---|---|
| n = 10 | n = 8 | |
| Gender (male/female) | 5/5 | 1/7 |
| Age (years), median (IQ range) | 68 (57–74) | 46 (27–68) |
| Underlying kidney disease | N.A. | |
| hypertensive nephropathy | 1 | |
| diabetic nephropathy | 2 | |
| polycystic kidney disease | 1 | |
| SLE-mediated glomerulonephritis | 1 | |
| granulomatous polyangiitis | 1 | |
| Nephronophthisis (type 1 homozygous mutation NPHP) | 1 | |
| Unknown | 1 | |
| Cause of transplant failure | N.A. | |
| Rejection | 5 | |
| chronic damage | 3 | |
| Months since transplantation, median (IQ range) | N.A. | 32 (21–52) |
| Number of immunosuppressive agents at time of sample collection, median (IQ range) | N.A. | 2 (1–2) |
| Donor type (living/deceased) | N.A. | 1/7 |
2.2. Isolation of Peripheral Blood Mononuclear Cells
2.3. Enzymatic Dissociation of Kidney Tissue
2.4. Explant Cultures of Kidney Tissue
2.5. Immunohistochemistry
2.6. Characterization of T Cell Subsets Obtained from Kidney Tissue
2.7. Characterization of TCR Vβ-Repertoire of T Cells Obtained from Kidney Tissue
2.8. Functional Characterization of T Cells Obtained from Kidney Tissue
2.9. BKV- and CMV-Reactive T Cells and Proliferation Potential of T Cells Obtained from Kidney Tissue
2.10. Statistical Analyses
3. Results
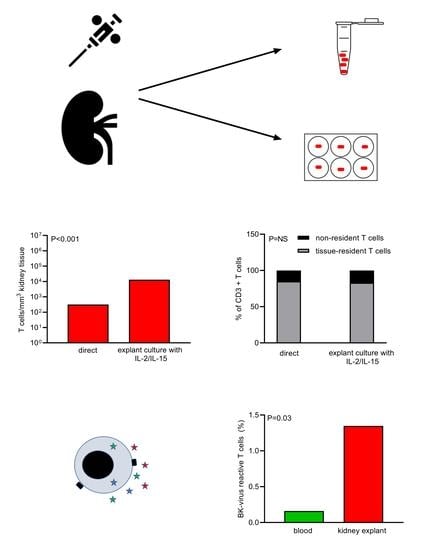
3.1. Lymphocytes Migrate from Kidney Tissue Cultures and Remain a Polyclonal Cell Population
3.2. Phenotype of Kidney T Cells after Tissue Culture Remains Largely Unchanged
3.3. Profiles of Cytokine- and CD107a—Expressing Kidney T Cells before and after Tissue Culture
3.4. CMV- and BKV-Reactive Kidney T Cells before and after Culture
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parga-Vidal, L.; van Aalderen, M.C.; Stark, R.; van Gisbergen, K.P.J.M. Tissue-resident memory T cells in the urogenital tract. Nat. Rev. Nephrol. 2022, 18, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Law, B.M.P.; Wilkinson, R.; Wang, X.; Kildey, K.; Giuliani, K.; Beagley, K.; Ungerer, J.; Healy, H.; Kassianos, A.J. Human Tissue-Resident Mucosal-Associated Invariant T (MAIT) Cells in Renal Fibrosis and CKD. J. Am. Soc. Nephrol. 2019, 30, 1322–1335. [Google Scholar] [CrossRef]
- Park, J.-G.; Na, M.; Kim, M.-G.; Park, S.H.; Lee, H.J.; Kim, D.K.; Kwak, C.; Kim, Y.S.; Chang, S.; Moon, K.C.; et al. Immune cell composition in normal human kidneys. Sci. Rep. 2020, 10, 15678. [Google Scholar] [CrossRef]
- Kildey, K.; Francis, R.S.; Hultin, S.; Harfield, M.; Giuliani, K.; Law, B.M.P.; Wang, X.; See, E.J.; John, G.; Ungerer, J.; et al. Specialized Roles of Human Natural Killer Cell Subsets in Kidney Transplant Rejection. Front. Immunol. 2019, 10, 1877. [Google Scholar] [CrossRef] [Green Version]
- Dornieden, T.; Sattler, A.; Pascual-Reguant, A.; Ruhm, A.H.; Thiel, L.G.; Bergmann, Y.S.; Thole, L.M.L.; Köhler, R.; Kühl, A.A.; Hauser, A.E.; et al. Signatures and Specificity of Tissue-Resident Lymphocytes Identified in Human Renal Peritumor and Tumor Tissue. J. Am. Soc. Nephrol. 2021, 32, 2223–2241. [Google Scholar] [CrossRef]
- Martina, M.N.; Noel, S.; Saxena, A.; Bandapalle, S.; Majithia, R.; Jie, C.; Arend, L.J.; Allaf, M.E.; Rabb, H.; Hamad, A.R.A. Double-Negative αβ T Cells Are Early Responders to AKI and Are Found in Human Kidney. J. Am. Soc. Nephrol. 2015, 27, 1113–1123. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Sato, Y.; Saito, M.; Fukuma, S.; Saito, M.; Yamamoto, S.; Komatsuda, A.; Fujiyama, N.; Satoh, S.; Lee, S.-H.; et al. Advanced Tertiary Lymphoid Tissues in Protocol Biopsies are Associated with Progressive Graft Dysfunction in Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 33, 186–200. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Zhang, N.; Marshall, H.D.; Staron, M.M.; Guan, T.; Hu, Y.; Cauley, L.S.; Craft, J.; Kaech, S.M. CD4+ T Cell Help Guides Formation of CD103+ Lung-Resident Memory CD8+ T Cells during Influenza Viral Infection. Immunity 2014, 41, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Abou-Daya, K.I.; Tieu, R.; Zhao, D.; Rammal, R.; Sacirbegovic, F.; Williams, A.L.; Shlomchik, W.D.; Oberbarnscheidt, M.H.; Lakkis, F.G. Resident memory T cells form during persistent antigen exposure leading to allograft rejection. Sci. Immunol. 2021, 6, eabc8122. [Google Scholar] [CrossRef]
- Turner, D.L.; Gordon, C.L.; Farber, D.L. Tissue-resident T cells, in situ immunity and transplantation. Immunol. Rev. 2014, 258, 150–166. [Google Scholar] [CrossRef]
- Son, Y.M.; Cheon, I.S.; Wu, Y.; Chaofan, L.; Zheng, W.; Xiaochen, G.; Yao, C.; Yoshimasa, T.; Yang-Xin, F.; Alexander, L.D.; et al. Tissue-resident CD4(+) T helper cells assist the development of protective respiratory B and CD8(+) T cell memory responses. Sci. Immunol. 2021, 6, eabb6852. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Daccache, J.; Jain, D.; Ko, K.; Kinloch, A.; Veselits, M.; Wolfgeher, D.; Chang, A.; Josephson, M.; Cunningham, P.; et al. Innate-like self-reactive B cells infiltrate human renal allografts during transplant rejection. Nat. Commun. 2021, 12, 4372. [Google Scholar] [CrossRef]
- Kamburova, E.G.; Gruijters, M.L.; Kardol-Hoefnagel, T.; Bram, W.B.; Joosten, W.I.; Allebes, W.A.; van der Meer, A.; Hilbrands, L.B.; Baas, M.C.; Spierings, E.; et al. Antibodies against ARHGDIB are associated with long-term kidney graft loss. Am. J. Transplant. 2019, 19, 3335–3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betjes, M.G.; Sablik, K.A.; Litjens, N.H.; Otten, H.G.; de Weerd, A.E. ARHGDIB and AT1R autoantibodies are differentially related to the development and presence of chronic antibody-mediated rejection and fibrosis in kidney allografts. Hum. Immunol. 2021, 82, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef]
- Pope, M.; Betjes, M.; Romani, N.; Hirmand, H.; Cameron, P.; Hoffman, L.; Gezelter, S.; Schuler, G.; Steinman, R. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 1994, 78, 389–398. [Google Scholar] [CrossRef]
- Pope, M.; Betjes, M.G.; Hirmand, H.; Hoffinan, L.; Steinman, R.M. Both Dendritic Cells and Memory T Lymphocytes Emigrate From Organ Cultures of Human Skin and Form Distinctive Dendritic-T-Cell Conjugates. J. Investig. Dermatol. 1995, 104, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.A.; Chong, B.F.; Mirchandani, N.; Yamanaka, K.-I.; Murphy, G.F.; Dowgiert, R.K.; Kupper, T.S. A Novel Method for the Isolation of Skin Resident T Cells from Normal and Diseased Human Skin. J. Investig. Dermatol. 2006, 126, 1059–1070. [Google Scholar] [CrossRef] [Green Version]
- Litjens, N.H.; van de Wetering, J.; van Besouw, N.M.; Betjes, M.G.H. The human alloreactive CD4+ T-cell repertoire is biased to a Th17 response and the frequency is inversely related to the number of HLA class II mismatches. Blood 2009, 114, 3947–3955. [Google Scholar] [CrossRef]
- Habib, S.M.; Abrahams, A.C.; Korte, M.; Zietse, R.; De Vogel, L.; Boer, W.; Dendooven, A.; Groningen, M.C.-V.; Betjes, M. CD4-Positive T Cells and M2 Macrophages Dominate the Peritoneal Infiltrate of Patients with Encapsulating Peritoneal Sclerosis. PLoS ONE 2015, 10, e0120174. [Google Scholar] [CrossRef] [Green Version]
- Betjes, M.; Habib, M.S.; Struijk, D.G.; Barreto, D.L.; Korte, M.R.; Abrahams, A.C.; Nagtzaam, N.M.; Groningen, M.C.C.-V.; Dik, W.A.; Litjens, N. Encapsulating peritoneal sclerosis is associated with T-cell activation. Nephrol. Dial. Transplant. 2015, 30, 1568–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, D.A.; da Silva, H.B.; Beura, L.K.; Peng, C.; Hamilton, S.E.; Masopust, D.; Jameson, S.C. The Functional Requirement for CD69 in Establishment of Resident Memory CD8+T Cells Varies with Tissue Location. J. Immunol. 2019, 203, 946–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Berg, P.J.; Hoevenaars, E.C.; Yong, S.L.; Karlijn, I.K.A.M.; van Donselaar-van der Pantvan, K.A.M.I.; van Tellingen, A.; Florquin, S.; van Lier, R.A.W.; Bemelman, F.J.; Berge, I.J.M.T. Circulating lymphocyte subsets in different clinical situations after renal transplantation. Immunology 2012, 136, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Dedeoglu, B.; Meijers, R.W.J.; Klepper, M.; Hesselink, D.A.; Baan, C.C.; Litjens, N.H.R.; Betjes, M.G.H. Loss of CD28 on Peripheral T Cells Decreases the Risk for Early Acute Rejection after Kidney Transplantation. PLoS ONE 2016, 11, e0150826. [Google Scholar] [CrossRef] [Green Version]
- Dedeoglu, B.; de Weerd, A.E.; Huang, L.; Langerak, A.W.; Dor, F.J.; Klepper, M.; Verschoor, W.; Reijerkerk, D.; Baan, C.C.; Litjens, N.H.R.; et al. Lymph node and circulating T cell characteristics are strongly correlated in end-stage renal disease patients, but highly differentiated T cells reside within the circulation. Clin. Exp. Immunol. 2017, 188, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Betjes, M.G.H. Clinical consequences of circulating CD28-negative T cells for solid organ transplantation. Transpl. Int. 2015, 29, 274–284. [Google Scholar] [CrossRef]
- Huang, L.; Betjes, M.G.H.; Klepper, M.; Anton, W.L.; Carla, C.B.; Nicolle, H.R.L. End-Stage Renal Disease Causes Skewing in the TCR Vbeta-Repertoire Primarily within CD8(+) T Cell Subsets. Front. Immunol. 2017, 8, 1826. [Google Scholar] [CrossRef] [Green Version]
- Betjes, M.G.H.; Langerak, A.W.; van der Spek, A.; de Wit, E.A.; Litjens, N.H.R. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. 2011, 80, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Smolders, J.; Heutinck, K.M.; Fransen, N.L.; Remmerswaal, E.B.M.; Hombrink, P.; Berge, I.J.M.T.; Van Lier, R.A.W.; Huitinga, I.; Hamann, J. Tissue-resident memory T cells populate the human brain. Nat. Commun. 2018, 9, 4593. [Google Scholar] [CrossRef] [Green Version]
- Ogongo, P.; Tezera, L.B.; Ardain, A.; Nhamoyebonde, S.; Ramsuran, D.; Singh, A.; Ng’Oepe, A.; Karim, F.; Naidoo, T.; Khan, K.; et al. Tissue-resident-like CD4+ T cells secreting IL-17 control Mycobacterium tuberculosis in the human lung. J. Clin. Investig. 2021, 131, e142014. [Google Scholar] [CrossRef]
- Litjens, N.H.R.; de Wit, E.A.; Baan, C.C.; Betjes, M.G.H. Activation-induced CD137 is a fast assay for identification and multi-parameter flow cytometric analysis of alloreactive T cells. Clin. Exp. Immunol. 2013, 174, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Dedeoglu, B.; Litjens, N.H.R.; Klepper, M.; Kraaijeveld, R.; Verschoor, W.; Baan, C.C.; Betjes, M.G.H. CD4+ CD28null T cells are not alloreactive unless stimulated by interleukin-15. Am. J. Transplant. 2017, 18, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Putten, C.; Remmerswaal, E.B.M.; Terpstra, M.L.; van der Bom, N.D.; Kers, J.; Berge, I.J.M.T.; Geerlings, S.E.; van Lier, R.A.W.; Bemelman, F.J.; van Aalderen, M.C. CD8 and CD4 T Cell Populations in Human Kidneys. Cells 2021, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Duquesnoy, R.J.; Trager, J.D.; Zeevi, A. Propagation and characterization of lymphocytes from transplant biopsies. Crit. Rev. Immunol. 1991, 10, 455–480. [Google Scholar]
- Engstrand, M.; Larsson, E.; Naghibi, M.; Tufveson, G.; Korsgren, O.; Johnsson, C. Lymphocyte propagation from biopsies of kidney allografts. Transpl. Immunol. 2006, 16, 215–219. [Google Scholar] [CrossRef]
- Mayer, T.G.; Fuller, A.A.; Fuller, T.C.; Lazarovits, A.I.; Boyle, L.A.; Kurnick, J.T. Characterization of in vivo-activated allospecific T lymphocytes propagated from human renal allograft biopsies undergoing rejection. J. Immunol. 1985, 134, 258–264. [Google Scholar]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Sablik, K.A.; Jordanova, E.S.; Pocorni, N.; Groningen, M.C.C.-V.; Betjes, M.G.H. Immune Cell Infiltrate in Chronic-Active Antibody-Mediated Rejection. Front. Immunol. 2020, 10, 3106. [Google Scholar] [CrossRef] [Green Version]
- Corrado, M.; Pearce, E.L. Targeting memory T cell metabolism to improve immunity. J. Clin. Investig. 2022, 132, e148546. [Google Scholar] [CrossRef]







| Median and IQ Range n = 8 Kidney Tissues | Lymphocyte Population | Resident (CD69+CD103+/−) (%) | Passenger (CD69−CD103−/+) (%) |
|---|---|---|---|
| T cells (cells/mm3) median | 317 (56–1269) | 87.24 (76.28–94.30) | 12.77 (5.71–23.71) |
| CD4+T cells (% of CD3+ T cells) | 47.71 (26.68–59.16) | 89.64 (76.64–94.64) | 10.37 (5.37–23.36) |
| CD8+T cells (% of CD3+ T cells) | 45.55 (31.40–65.00) | 88.45 (81.38–95.55) | 11.56 (4.44–18.63) |
| CD4−CD8−T cells (% of CD3+ T cells) | 4.98 (3.06–7.02) | 81.29 (73.76–89.4) | 18.72 (10.61–26.25) |
| CD161+T cells (% of CD3+ T cells) | 14.55 (10.35–17.25) | 95.13 (91.73–98.7) | 4.88 (1.30–8.27) |
| NK cells (cells/mm3) | 12 (8–81) | 76.84 (64.01–90.53) | 23.17 (9.47–35.99) |
| Resident (CD69+CD103+/−) (%) | Passenger (CD69−CD103−/+) (%) | p ** | |
|---|---|---|---|
| CD4+T cell subsets | |||
| Naive (CD45RA+CCR7+) * | 1.54 (0.23–3.46) | 3.72 (0.33–6.71) | 0.02 |
| CM (CD45RA−CCR7+) * | 9.06 (6.70–36.43) | 8.76 (6.07–40.52) | 0.84 |
| EM (CD45RA−CCR7−) * | 89.14 (55.99–90.07) | 85.04 (47.16–89.27) | <0.01 |
| EMRA (CD45RA+CCR7−) * | 1.99 (1.13–3.41) | 4.14 (2.56–6.93) | <0.01 |
| CD27+CD28+ * | 30.18 (9.35–33.23) | 14.30 (4.47–26.07) | <0.01 |
| CD27−CD28+ * | 58.11 (50.12–82.09) | 63.32 (34.30–85.07) | 1.00 |
| CD27+CD28− * | 0.78 (0.15–1.39) | 0.67 (0.15–1.63) | 0.84 |
| CD27−CD28− * | 7.42 (5.09–15.67) | 12.05 (7.08–40.22) | 0.02 |
| Tregs (CD45RA+/−FOXP3+) * | 2.06 (0.91–2.42) | 1.39 (0.72–2.66) | 0.83 |
| Tbet+ | 72.86 (57.93–88.21) | 63.76 (35.21–83.44) | 0.08 |
| RORgamma+ | 2.21 (1.89–3.72) | 5.22 (2.91–9.14) | 0.02 |
| FOXP3+ | 5.34 (2.91–9.14) | 3.87 (1.89–5.81) | 0.04 |
| CD8+T cell subsets | |||
| Naive (CD45RA+CCR7+) * | 1.65 (0.81–3.54) | 3.78 (0.83–6.32) | 0.11 |
| CM (CD45RA−CCR7+) * | 4.31 (3.56–6.82) | 5.32 (2.70–17.12) | 0.38 |
| EM (CD45RA−CCR7−) * | 78.70 (60.88–83.88) | 45.92 (37.16–69.45) | <0.01 |
| EMRA (CD45RA+CCR7−) * | 14.54 (7.44–31.61) | 24.93 (15.98–50.04) | 0.05 |
| CD27+CD28+ * | 18.69 (5.10–38.65) | 3.54 (0.83–13.51) | <0.01 |
| CD27−CD28+ * | 14.85 (5.92–42.66) | 17.12 (5.81–30.68) | 0.84 |
| CD27+CD28− * | 21.59 (4.43–43.67) | 7.84 (0.38–17.17) | 0.08 |
| CD27−CD28− * | 30.62 (13.19–48.10) | 58.15 (31.55–75.33) | 0.04 |
| Tbet+ | 83.75 (77.12–91.85) | 88.61 (73.32–96.86) | 0.90 |
| RORγ+ | 1.57 (0.70–3.51) | 3.83 (2.34–9.45) | <0.01 |
| FOXP3+ | 0.97 (0.33–1.41) | 1.95 (1.46–6.93) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betjes, M.G.H.; Prevoo, F.; van den Bosch, T.P.P.; Klepper, M.; Litjens, N.H.R. A Novel Technique for the Generation of Substantial Numbers of Functional Resident T Cells from Kidney Tissue. Cells 2022, 11, 2233. https://doi.org/10.3390/cells11142233
Betjes MGH, Prevoo F, van den Bosch TPP, Klepper M, Litjens NHR. A Novel Technique for the Generation of Substantial Numbers of Functional Resident T Cells from Kidney Tissue. Cells. 2022; 11(14):2233. https://doi.org/10.3390/cells11142233
Chicago/Turabian StyleBetjes, Michiel G. H., Frederique Prevoo, Thierry P. P. van den Bosch, Mariska Klepper, and Nicolle H. R. Litjens. 2022. "A Novel Technique for the Generation of Substantial Numbers of Functional Resident T Cells from Kidney Tissue" Cells 11, no. 14: 2233. https://doi.org/10.3390/cells11142233
APA StyleBetjes, M. G. H., Prevoo, F., van den Bosch, T. P. P., Klepper, M., & Litjens, N. H. R. (2022). A Novel Technique for the Generation of Substantial Numbers of Functional Resident T Cells from Kidney Tissue. Cells, 11(14), 2233. https://doi.org/10.3390/cells11142233