Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements
Abstract
:1. Introduction
2. Skin Architecture and Functions
3. Wounds and Wound Healing Process
4. Conventional Approaches Employed for Wound Healing
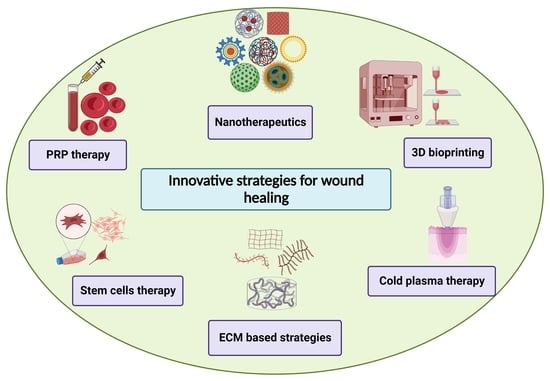
5. Innovative Strategies for Wound Healing
5.1. Nanotherapeutics-Based Strategies
| Type of Nanomaterials | Wound Type | Drugs/Therapeutic Agents/Growth Factors | Findings | Reference |
|---|---|---|---|---|
| Poly (ethylene terephthalate) (PET) nanofibers | Acute (skin wound) | Anionic antibiotics piperacillin/tazobactam (PT) | High loading efficiency and sustained delivery for PT, reduced bacterial load | [116] |
| Poly (lactic-co-glycolic acid)/gelatin (PLGA)/gelatin nanofibers | Chronic (diabetic wound) | Liraglutide (Lira) | Shorter wound closure time, enhanced collagen deposition and alignment, increased blood vessel density | [117] |
| Poly (lactic-co-glycolic acid)-polyethylenimine nanoparticles | Acute (skin wound) | Nitric oxide (NO) | Strong bactericidal effect against methicillin-resistant Staphylococcus aureus (MRSA) bacteria, accelerated wound healing | [118] |
| α-gal nanoparticles | Chronic (diabetic wound) | ----------------------- | Enhanced vascularization, re-epithelialization, granulation tissue formation, accelerated wound healing | [119] |
| Solid lipid nanoparticles | Chronic wound | Serpin A1 (A1) and host defense peptide LL37 | Promotion of wound closure, reduction of bacterial contamination, and enhancement of anti-inflammatory activity | [120] |
| Liposome with silk fibroin hydrogels | Chronic (deep second-degree scald) | Basic fibroblast growth factor (bFGF) | Accelerated the wound closure, induced regeneration of vascular vessel | [121] |
| Photoluminescent gold nanodots | Acute (skin wound) | Antimicrobial peptide (surfactin; SFT), and 1-dodecanethiol (DT) | Enhanced antimicrobial properties and collagen deposition | [122] |
| Peptide dendrimers | Chronic (diabetic wound) | ----------------------- | Smaller wound area percentage, improved wound healing | [123] |
| Fusidic acid nanoemulsion | Chronic (burn wound) | ----------------------- | Reduction in bacterial load, wound contraction, and faster re-epithelialization | [124] |
| Recombinant human hair keratin nanoparticles | Acute (dermal wound) | ----------------------- | Improved epithelialization, vascularization, along with collagen deposition and remodeling. | [125] |
| Chitosan nanoparticles | Chronic (prostatic wound) | Rebamipide | Improved re-epithelialization and faster wound healing | [126] |
| PLGA-liposome nanofibers | Acute (skin wound) | MicroRNA 145 (miR-145) and platelet-derived growth factor (PDGF) | Promotion of wound healing with enhanced vascularization and decreased wound size | [127] |
| Gelatin nanofibers | Chronic (burn wound) | anionic drug and hydrotalcite | Accelerated wound healing with strong antimicrobial activity | [128] |
| Silk fibroin nanoparticles | Chronic (ulcerative colitis) | Resveratrol | Reduced level of intracellular ROS, polarization of macrophages to type M2, restoration of damaged colonic epithelial barriers, reduced inflammatory reactions and level of intracellular ROS. | [129] |
| Poly (l-lactic acid) (PLLA) nanofibers | Chronic (diabetic wound) | Silica nanoparticles and dimethyloxalylglycine | Improved neo-vascularization and re-epithelialization with enhanced collagen deposition | [130] |
| Poly-(1,4-phenyleneacetone dimethylene thioketal) | Acute (full-thickness skin defect) | Stromal cell-derived factor-1α(SDF-1α) | Induction of wound vascularization, accelerated wound healing | [131] |
| Elastic liposomes with hyaluronic acid | Chronic (diabetic wound) | Epidermal growth factor (EGF), platelet-derived growth factor-A (PDGF-A), and insulin-like growth factor-I (IGF-I) | Reduction of wound size, improved skin permeation, and healing | [132] |
| Chitosan capped silver nanoparticles | Chronic (burn wound) | ----------------------- | Shortening of the length of repair phases, enhanced re-epithelialization | [133] |
| Polyvinyl alcohol nanogels | Acute (skin wound) | Cerium oxide nanoparticles | Antimicrobial activity and rapid healing | [111] |
| Copper nanoparticles | Chronic wound | ----------------------- | Increased vascularization, accelerated healing process | [134] |
| Chitosan hydrogels | Chronic (diabetic wound) | Silver nanoparticles | Promotion of antibacterial activity, enhanced healing | [135] |
| Polymeric composite dressings | Chronic (diabetic wound) | Calcium | Stimulated angiogenesis, collagen synthesis, accelerated wound healing | [136] |
| Fibrin nanoparticles | Acute (dermal wound) | Keratinocyte growth factor | Better cell proliferation and migration along with enhanced wound healing | [137] |
| Chitosan/Collagen blended nanofibers | Acute (full thickness skin wound) | Curcumin | Reduction in wound coverage area, improved healing | [138] |
| Collagen mats | Chronic wound | Inorganic polyphosphate (polyP) | Reduction in wound area, accelerated re-epithelialization rate and healing | [139] |
5.2. Stem Cell Therapy-Based Strategies
5.3. D Bioprinting-Based Strategies
5.4. Extracellular Matrix (ECM)-Based Strategies
5.5. Platelet-Rich Plasma (PRP)-Based Strategies
5.6. Cold Atmospheric Plasma Therapy-Based Strategies
5.7. MicroRNA (miR)-Based Strategy for Wound Healing
6. Challenges and Future Prospective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Díaz-García, D.; Filipová, A.; Garza-Veloz, I.; Martinez-Fierro, M.L. A Beginner’s Introduction to Skin Stem Cells and Wound Healing. Int. J. Mol. Sci. 2021, 22, 11030. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, X.; Zheng, H.; Tang, Y.; Zeng, K.; Shao, L.; Li, L. Nanomaterials applied in wound healing: Mechanisms, limitations and perspectives. J. Control Release 2021, 337, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Dekoninck, S.; Rulands, S.; Lenglez, S.; Mascré, G.; Simons, B.; Blanpain, C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017, 8, 14684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Swanson, T.; Keast, D.; Cooper, R.; Black, J.; Angel, D.; Schultz, G.; Carville, K.; Fletcher, J.J.W.I. Ten top tips: Identification of wound infection in a chronic wound. Wounds Int. 2015, 6, 22–27. [Google Scholar]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, D.G.; Swerdlow, M.A.; Armstrong, A.A.; Conte, M.S.; Padula, W.V.; Bus, S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot Ankle Res. 2020, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Jeffcoate, W.J.; Vileikyte, L.; Boyko, E.J.; Armstrong, D.G.; Boulton, A.J. Current Challenges and Opportunities in the Prevention and Management of Diabetic Foot Ulcers. Diabetes Care 2018, 41, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-Z.; Gou, M.; Da, L.-C.; Zhang, M.W.-Q.; Xie, H.-Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef]
- Monavarian, M.; Kader, S.; Moeinzadeh, S.; Jabbari, E. Regenerative Scar-Free Skin Wound Healing. Tissue Eng. Part B Rev. 2019, 25, 294–311. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Okur, N.; Siafaka, P.I. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef]
- Sen, C.K. Human wound and its burden: Updated 2020 compendium of estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Shafiee, A. Engineering Bioactive Scaffolds for Skin Regeneration. Small 2021, 17, 2101384. [Google Scholar] [CrossRef] [PubMed]
- Mascré, G.; Dekoninck, S.; Drogat, B.; Youssef, K.K.; Brohée, S.; Sotiropoulou, P.A.; Simons, B.D.; Blanpain, C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 2012, 489, 257–262. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Hou, Y.; Chai, J.; Duan, H.; Chu, W.; Zhang, H.; Hu, Q.; Du, J. Human Umbilical Cord Mesenchymal Stem Cells Transplantation Promotes Cutaneous Wound Healing of Severe Burned Rats. PLoS ONE 2014, 9, e88348. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Kim, M.J.; Ki, C.S.; Kim, H.J.; Park, Y.H. Fabrication of bi-layer scaffold of keratin nanofiber and gelatin-methacrylate hydrogel: Implications for skin graft. Int. J. Biol. Macromol. 2017, 105, 541–548. [Google Scholar] [CrossRef]
- Lorenz, H.P.; Leavitt, T.; Hu, M.S.; Marshall, C.D.; A Barnes, L.; Longaker, M.T. Stem cells and chronic wound healing: State of the art. Chronic Wound Care Manag. Res. 2016, 3, 7–27. [Google Scholar] [CrossRef] [Green Version]
- Krisp, C.; Jacobsen, F.; McKay, M.J.; Molloy, M.P.; Steinstraesser, L.; Wolters, D.A. Proteome analysis reveals antiangiogenic environments in chronic wounds of diabetes mellitus type 2 patients. Proteomics 2013, 13, 2670–2681. [Google Scholar] [CrossRef]
- Chang, H.-K.; Kim, P.-H.; Cho, H.-M.; Yum, S.-Y.; Choi, Y.-J.; Son, Y.; Lee, D.; Kang, I.; Kang, K.-S.; Jang, G.; et al. Inducible HGF-secreting Human Umbilical Cord Blood-derived MSCs Produced via TALEN-mediated Genome Editing Promoted Angiogenesis. Mol. Ther. 2016, 24, 1644–1654. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Qiu, L.; Hu, W.; Deng, X.; Xu, H.; Cao, Y.; Xiao, Z.; Peng, L.; Johnson, S.; Alexey, L.; et al. Genetically-modified bone mesenchymal stem cells with TGF-β 3 improve wound healing and reduce scar tissue formation in a rabbit model. Exp. Cell Res. 2018, 367, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.M.; Briquez, P.S.; Ranga, A.; Lutolf, M.P.; Hubbell, J.A. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc. Natl. Acad. Sci. USA 2013, 110, 4563–4568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Liang, P.; Jiang, B.; Zhang, P.; Yu, W.; Duan, M.; Guo, L.; Cui, X.; Huang, M.; Huang, X. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020, 259, 118246. [Google Scholar] [CrossRef]
- Norouzi, M.; Boroujeni, S.M.; Omidvarkordshouli, N.; Soleimani, M. Advances in Skin Regeneration: Application of Electrospun Scaffolds. Adv. Healthc. Mater. 2015, 4, 1114–1133. [Google Scholar] [CrossRef]
- Ter Horst, B.; Chouhan, G.; Moiemen, N.S.; Grover, L.M. Advances in keratinocyte delivery in burn wound care. Adv. Drug Deliv. Rev. 2017, 123, 18–32. [Google Scholar] [CrossRef]
- Phua, Q.H.; Han, H.A.; Soh, B.-S. Translational stem cell therapy: Vascularized skin grafts in skin repair and regeneration. J. Transl. Med. 2021, 19, 83. [Google Scholar] [CrossRef]
- Agarwal, P.; Kukrele, R.; Sharma, D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: A review. J. Clin. Orthop. Trauma 2019, 10, 845–848. [Google Scholar] [CrossRef]
- Ashrafi, M.; Alonso-Rasgado, T.; Baguneid, M.; Bayat, A.J.E.D. The efficacy of electrical stimulation in lower extremity cutaneous wound healing: A systematic review. Exp. Dermatol. 2017, 26, 171–178. [Google Scholar] [CrossRef]
- Huang, C.; Leavitt, T.; Bayer, L.R.; Orgill, D.P. Effect of negative pressure wound therapy on wound healing. Curr. Probl. Surg. 2014, 51, 301–331. [Google Scholar] [CrossRef] [Green Version]
- Kloth Luther, C. Discussion: Advanced technologies to improve wound healing: Electrical stimulation, vibration therapy, and ultrasound—What is the evidence? Plast. Reconstr. Surg. 2016, 138, 105S–106S. [Google Scholar] [CrossRef]
- Vu, N.B.; Nguyen, H.T.; Palumbo, R.; Pellicano, R.; Fagoonee, S.; Pham, P.V. Stem cell-derived exosomes for wound healing: Current status and promising directions. Minerva Med. 2020, 112, 384–400. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Castaño, O.; Mateos-Timoneda, M.; Engel, E.; Pérez-Amodio, S. Nanotechnology Approaches in Chronic Wound Healing. Adv. Wound Care 2021, 10, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Semba, J.A.; Kulpa, A.; Dańczak-Pazdrowska, A.; Rybka, J.D.; Gornowicz-Porowska, J. 3D Bioprinting in Skin Related Research: Recent Achievements and Application Perspectives. ACS Synth. Biol. 2021, 11, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Kucharzewski, M.; Rojczyk, E.; Wilemska-Kucharzewska, K.; Wilk, R.; Hudecki, J.; Los, M.J. Novel trends in application of stem cells in skin wound healing. Eur. J. Pharmacol. 2018, 843, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, Y.-X.; Li, Y.-M. Generation of Skin Organoids: Potential Opportunities and Challenges. Front. Cell Dev. Biol. 2021, 9, 3176. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Natarajan, V.T.; Ganju, P.; Ramkumar, A.; Grover, R.; Gokhale, R.S. Multifaceted pathways protect human skin from UV radiation. Nat. Chem. Biol. 2014, 10, 542–551. [Google Scholar] [CrossRef]
- Menon, G.K.; Dryer, L.; Kalafsky, R. Approaches to the Development of Cosmetic Products to Counter the Effects of Skin Aging. In Skin Aging Handbook; William Andrew Publishing: Norwich, NY, USA; Elsevier: Amsterdam, The Netherlands, 2009; pp. 265–290. [Google Scholar]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef]
- Shirakata, Y. Regulation of epidermal keratinocytes by growth factors. J. Dermatol. Sci. 2010, 59, 73–80. [Google Scholar] [CrossRef]
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.L.; Mansbridge, J.N.; Carter, W.G.; Fujita, M.; Olerud, J.E. Keratinocyte Migration, Proliferation, and Differentiation in Chronic Ulcers from Patients with Diabetes and Normal Wounds. J. Histochem. Cytochem. 2008, 56, 687–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, C.T.; Hiroyasu, S.; Granville, D.J. Granzyme B as a therapeutic target for wound healing. Expert Opin. Ther. Targets 2019, 23, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Pirri, C.; Fede, C.; Fan, C.; Giordani, F.; Stecco, L.; Foti, C.; De Caro, R. Dermatome and fasciatome. Clin. Anat. 2019, 32, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A. Apostolos. Sebaceous lipids. In Lipids and Skin Health; Springer: Cham, Swtizerland, 2015; pp. 127–138. [Google Scholar]
- Stan, D.; Tanase, C.; Avram, M.; Apetrei, R.; Mincu, N.B.; Mateescu, A.L.; Stan, D.J.E.D. Wound healing applications of creams and “smart” hydrogels. Exp. Dermatol. 2021, 30, 1218–1232. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.M. Human Embryology and Developmental Biology; Elsevier Health Sciences: St.Lious, MO, USA, 2018. [Google Scholar]
- Brown, T.M.; Krishnamurthy, K. Histology, dermis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.-C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Iheanacho, F.; Vellipuram, A.R. Physiology, Mechanoreceptors; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Kim, J.; Simon, R. Calculated Decisions: Wound Closure Classification. Pediatr. Emerg. Med. Pr. 2018, 14, 1–3. [Google Scholar]
- Kuhlmann, M.; Wigger-Alberti, W.; Mackensen, Y.; Ebbinghaus, M.; Williams, R.; Krause-Kyora, F.; Wolber, R. Wound healing characteristics of a novel wound healing ointment in an abrasive wound model: A randomised, intra-individual clinical investigation. Wound Med. 2019, 24, 24–32. [Google Scholar] [CrossRef]
- Tort, S.; Demiröz, F.T.; Cevher, Ş.C.; Sarıbaş, S.; Özoğul, C.; Acartürk, F. The effect of a new wound dressing on wound healing: Biochemical and histopathological evaluation. Burns 2020, 46, 143–155. [Google Scholar] [CrossRef]
- Das, A.; Abas, M.; Biswas, N.; Banerjee, P.; Ghosh, N.; Rawat, A.; Khanna, S.; Roy, S.; Sen, C.K. A Modified Collagen Dressing Induces Transition of Inflammatory to Reparative Phenotype of Wound Macrophages. Sci. Rep. 2019, 9, 14293. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Liang, J.-X.; Li, S.-H.; Huang, S.; Yan, J.-X.; Xiao, L.-L.; Song, J.-X.; Liu, H.-W. Allogeneic Platelet-Rich Plasma Therapy as an Effective and Safe Adjuvant Method for Chronic Wounds. J. Surg. Res. 2019, 246, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Desmet, C.M.; Préat, V.; Gallez, B. Nanomedicines and gene therapy for the delivery of growth factors to improve perfusion and oxygenation in wound healing. Adv. Drug Deliv. Rev. 2018, 129, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhabra, S.; Chhabra, N.; Kaur, A.; Gupta, N. Wound Healing Concepts in Clinical Practice of OMFS. J. Maxillofac. Oral Surg. 2016, 16, 403–423. [Google Scholar] [CrossRef]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- El Ashram, S.; El-Samad, L.M.; Basha, A.A.; El Wakil, A. Naturally-derived targeted therapy for wound healing: Beyond classical strategies. Pharmacol. Res. 2021, 170, 105749. [Google Scholar] [CrossRef]
- Kanji, S.; Das, H. Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediat. Inflamm. 2017, 2017, 5217967. [Google Scholar] [CrossRef] [Green Version]
- Kawasumi, A.; Sagawa, N.; Hayashi, S.; Yokoyama, H.; Tamura, K. Wound Healing in Mammals and Amphibians: Toward Limb Regeneration in Mammals. Poxviruses 2012, 367, 33–49. [Google Scholar] [CrossRef]
- Wu, L.; Chen, X.; Zhao, J.; Martin, B.; Zepp, J.A.; Ko, J.S.; Gu, C.; Cai, G.; Ouyang, W.; Sen, G.; et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4–ERK5 axis. J. Exp. Med. 2015, 212, 1571–1587. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Barman, P.K.; Koh, T.J.J.F.i.C.; Biology, D. Macrophage dysregulation and impaired skin wound healing in diabetes. Front. Cell Dev. Biol. 2020, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Favara, G.; Lio, R.M.S.; Evola, G.; Agodi, A.; Basile, G. Nutrition and Wound Healing: An Overview Focusing on the Beneficial Effects of Curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekker, A.D.; Davis, F.M.; Kunkel, S.L.; Gallagher, K.A. Targeting epigenetic mechanisms in diabetic wound healing. Transl. Res. 2018, 204, 39–50. [Google Scholar] [CrossRef]
- Raja, S.K.; Garcia, M.S.; Isseroff, R.R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci.-Landmark 2007, 12, 2849–2868. [Google Scholar] [CrossRef] [Green Version]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmouliere, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Zhang, K.; Lui, V.C.H.; Chen, Y.; Lok, C.N.; Wong, K.K.Y. Delayed application of silver nanoparticles reveals the role of early inflammation in burn wound healing. Sci. Rep. 2020, 10, 5562. [Google Scholar] [CrossRef]
- Aydemir, I.; Ozturk, S.; Sönmez, P.K.; Tuğlu, M.I. Mesenchymal stem cells in skin wound healing. Anatomy 2016, 10, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Dowsett, C.; Newton, H. Wound bed preparation: TIME in practice. Wounds Uk 2005, 1, 58. [Google Scholar]
- Dowsett, C.; Gronemann, M.; Harding, K. Taking wound assessment beyond the edge. Wounds Int 2015, 6, 19–23. [Google Scholar]
- Gushiken, L.; Beserra, F.; Bastos, J.; Jackson, C.; Pellizzon, C. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Bellingeri, A.; Falciani, F.; Traspedini, P.; Moscatelli, A.; Russo, A.; Tino, G.; Chiari, P.; Peghetti, A. Effect of a wound cleansing solution on wound bed preparation and inflammation in chronic wounds: A single-blind RCT. J. Wound Care 2016, 25, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Lumbers, M. Wound debridement: Choices and practice. Br. J. Nurs. 2018, 27, S16–S20. [Google Scholar] [CrossRef] [PubMed]
- David, J.A.; Chiu, E.S. Surgical debridement. In Interventional Treatment of Wounds; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–15. [Google Scholar]
- Ferrario, C.; Rusconi, F.; Pulaj, A.; Macchi, R.; Landini, P.; Paroni, M.; Colombo, G.; Martinello, T.; Melotti, L.; Gomiero, C.; et al. From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine. Mar. Drugs 2020, 18, 414. [Google Scholar] [CrossRef]
- Varkey, M.; Ding, J.; Tredget, E.E. Advances in Skin Substitutes—Potential of Tissue Engineered Skin for Facilitating Anti-Fibrotic Healing. J. Funct. Biomater. 2015, 6, 547–563. [Google Scholar] [CrossRef]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef]
- Xu, K.; Chai, B.; Zhang, K.; Xiong, J.; Zhu, Y.; Xu, J.; An, N.; Xia, W.; Ji, H.; Wu, Y.; et al. Topical Application of Fibroblast Growth Factor 10-PLGA Microsphere Accelerates Wound Healing via Inhibition of ER Stress. Oxidative Med. Cell. Longev. 2020, 2020, 8586314. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Zeng, R.; Lin, C.; Lin, Z.; Chen, H.; Lu, W.; Lin, C.; Li, H. Approaches to cutaneous wound healing: Basics and future directions. Cell Tissue Res. 2018, 374, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Michelin, R.M.; Ahdoot, E.; Zakhary, B.L.; McDowell, M.; French, M. Choosing the Optimal Wound Dressing for Bathing after Total Knee Arthroplasty. J. Arthroplast. 2020, 36, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Cai, Z.; Ye, T.; Yu, X.; Chen, Z.; Yan, Y.; Qi, J.; Wang, L.; Liu, Z.; Cui, W.; et al. Injectable Polypeptide-Protein Hydrogels for Promoting Infected Wound Healing. Adv. Funct. Mater. 2020, 30, 2001196. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Y.; Pan, W.; Tong, X.; Zeng, Q.; Su, T.; Qi, X.; Shen, J. Polydopamine-incorporated dextran hydrogel drug carrier with tailorable structure for wound healing. Carbohydr. Polym. 2020, 253, 117213. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shi, M.; Liang, Y.; Guo, B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Goodarzi, P.; Falahzadeh, K.; Nematizadeh, M.; Farazandeh, P.; Payab, M.; Larijani, B.; Beik, A.T.; Arjmand, B. Tissue Engineered Skin Substitutes. J. Artif. Organs 2018, 1107, 143–188. [Google Scholar] [CrossRef]
- Still, J.; Glat, P.; Silverstein, P.; Griswold, J.; Mozingo, D. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns 2003, 29, 837–841. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Ma, J.; Ye, J.; Guo, P.; Li, L. Fate of antibiotic-resistant bacteria and antibiotic resistance genes in the electrokinetic treatment of antibiotic-polluted soil. Chem. Eng. J. 2018, 337, 584–594. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2017, 127, 46–57. [Google Scholar] [CrossRef]
- Jahromi, M.A.M.; Zangabad, P.S.; Basri, S.M.M.; Zangabad, K.S.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2017, 123, 33–64. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Rahman, M.; Kamal, M.A. Special issue: Cancer nanotherapeutics: Targeted medicine, therapeutic vaccination and challenges with cancer nanomedicines. Semin. Cancer Biol. 2021, 69, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Hu, J.; Qian, W.; Chen, L.; Zhang, D. Recent advances in nanotherapeutics for the treatment of burn wounds. Burn. Trauma 2021, 9, tkab026. [Google Scholar] [CrossRef] [PubMed]
- Malekzad, H.; Mirshekari, H.; Zangabad, P.S.; Basri, S.M.M.; Baniasadi, F.; Aghdam, M.S.; Karimi, M.; Hamblin, M.R. Plant protein-based hydrophobic fine and ultrafine carrier particles in drug delivery systems. Crit. Rev. Biotechnol. 2017, 38, 47–67. [Google Scholar] [CrossRef] [PubMed]
- Debone, H.S.; Lopes, P.S.; Severino, P.; Yoshida, C.M.P.; Souto, E.B.; da Silva, C.F. Chitosan/Copaiba oleoresin films for would dressing application. Int. J. Pharm. 2018, 555, 146–152. [Google Scholar] [CrossRef]
- Safdar, M.H.; Hussain, Z.; Abourehab, M.A.S.; Hasan, H.; Afzal, S.; Thu, H.E. New developments and clinical transition of hyaluronic acid-based nanotherapeutics for treatment of cancer: Reversing multidrug resistance, tumour-specific targetability and improved anticancer efficacy. Artif. Cell. Nanomed. Biotechnol. 2017, 46, 1967–1980. [Google Scholar] [CrossRef]
- Rahim, M.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z.; et al. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers 2021, 13, 670. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Rawas-Qalaji, M.; Naseem, M.; Khan, S.; Sohail, M. Recent developments and advanced strategies for promoting burn wound healing. J. Drug Deliv. Sci. Technol. 2022, 68, 103092. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Lu, C.-T.; Zhang, Y.; Xiao, J.; Zhao, Y.-P.; Tian, J.-L.; Xu, Y.-Y.; Feng, Z.-G.; Xu, C.-Y. Selection of high efficient transdermal lipid vesicle for curcumin skin delivery. Int. J. Pharm. 2013, 454, 302–309. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Mishra, A.; Sharma, D.; Singh, K. Antiviral and antimicrobial potentiality of nano drugs. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 343–356. [Google Scholar]
- Gabrielyan, L.; Hovhannisyan, A.; Gevorgyan, V.; Ananyan, M.; Trchounian, A. Antibacterial effects of iron oxide (Fe3O4) nanoparticles: Distinguishing concentration-dependent effects with different bacterial cells growth and membrane-associated mechanisms. Appl. Microbiol. Biotechnol. 2019, 103, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Baker, A.B. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Shao, G.; Ren, F.; Yang, M.; Nie, Y.; Peng, Q.; Zhang, P. Cerium oxide nanoparticle-loaded polyvinyl alcohol nanogels delivery for wound healing care systems on surgery. Drug Deliv. 2021, 28, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.; Ghaffari, A.; Rashidzadeh, H.; Momeni badeleh, S.; Maleki, Y. Current status and future outlook of nano-based systems for burn wound management. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1934–1952. [Google Scholar] [CrossRef]
- Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1551–1573. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Zak, M.S.; Majdi, H.; Mostafavi, E.; Barati, M.; Lotfimehr, H.; Ghaseminasab, K.; Pazoki-Toroudi, H.; Webster, T.J.; Akbarzadeh, A. The effect of chrysin–curcumin-loaded nanofibres on the wound-healing process in male rats. Artif. Cell. Nanomed. Biotechnol. 2019, 47, 1642–1652. [Google Scholar] [CrossRef]
- Wasef, L.G.; Shaheen, H.M.; El-Sayed, Y.S.; Shalaby, T.I.A.; Samak, D.H.; El-Hack, M.E.A.; Al-Owaimer, A.; Saadeldin, I.M.; El-Mleeh, A.; Ba-Awadh, H.; et al. Effects of Silver Nanoparticles on Burn Wound Healing in a Mouse Model. Biol. Trace Element Res. 2019, 193, 456–465. [Google Scholar] [CrossRef]
- Liu, S.; Fukushima, K.; Venkataraman, S.; Hedrick, J.L.; Yang, Y.Y. Supramolecular nanofibers self-assembled from cationic small molecules derived from repurposed poly(ethylene teraphthalate) for antibiotic delivery. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 165–172. [Google Scholar] [CrossRef]
- Yu, M.; Huang, J.; Zhu, T.; Lu, J.; Liu, J.; Li, X.; Yan, X.; Liu, F. Liraglutide-loaded PLGA/gelatin electrospun nanofibrous mats promote angiogenesis to accelerate diabetic wound healing via the modulation of miR-29b-3p. Biomater. Sci. 2020, 8, 4225–4238. [Google Scholar] [CrossRef]
- Nurhasni, H.; Cao, J.; Choi, M.; Kim, I.; Lee, B.L.; Jung, Y.; Yoo, J.-W. Nitric oxide-releasing poly (lactic-co-glycolic acid)-polyethylenimine nanoparticles for prolonged nitric oxide release, antibacterial efficacy, and in vivo wound healing activity. Int. J. Nanomed. 2015, 10, 3065. [Google Scholar]
- Kaymakcalan, O.E.; Abadeer, A.; Goldufsky, J.W.; Galili, U.; Karinja, S.J.; Dong, X.; Jin, J.L.; Samadi, A.; Spector, J.A. Topical α-gal nanoparticles accelerate diabetic wound healing. Exp. Dermatol. 2020, 29, 404–413. [Google Scholar] [CrossRef]
- Fumakia, M.; Ho, E.A. Nanoparticles encapsulated with LL37 and serpin A1 promotes wound healing and synergistically enhances antibacterial activity. Mol. Pharm. 2016, 13, 2318–2331. [Google Scholar] [CrossRef]
- Xu, H.-L.; Chen, P.-P.; Zhuge, D.-L.; Zhu, Q.-Y.; Jin, B.-H.; Shen, B.-X.; Xiao, J.; Zhao, Y.-Z. Liposomes with Silk Fibroin Hydrogel Core to Stabilize bFGF and Promote the Wound Healing of Mice with Deep Second-Degree Scald. Adv. Healthc. Mater. 2017, 6, 1700344. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Chang, H.-Y.; Lu, J.-K.; Huang, Y.-C.; Harroun, S.G.; Tseng, Y.-T.; Li, Y.-J.; Huang, C.-C.; Chang, H.-T. Self-Assembly of Antimicrobial Peptides on Gold Nanodots: Against Multidrug-Resistant Bacteria and Wound-Healing Application. Adv. Funct. Mater. 2015, 25, 7189–7199. [Google Scholar] [CrossRef]
- Deng, X.; Li, X.; Chen, W.; Zhao, T.; Huang, W.; Qian, H. Design, synthesis and biological evaluation of peptide dendrimers with wound healing promoting activity. Med. Chem. Res. 2017, 26, 580–586. [Google Scholar] [CrossRef]
- Thakur, K.; Sharma, G.; Singh, B.; Jain, A.; Tyagi, R.; Chhibber, S.; Katare, O.P. Cationic-bilayered nanoemulsion of fusidic acid: An investigation on eradication of methicillin-resistant Staphylococcus aureus 33591 infection in burn wound. Nanomedicine 2018, 13, 825–847. [Google Scholar] [CrossRef]
- Gao, F.; Li, W.; Deng, J.; Kan, J.; Guo, T.; Wang, B.; Hao, S. Recombinant Human Hair Keratin Nanoparticles Accelerate Dermal Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 18681–18690. [Google Scholar] [CrossRef]
- Sun, M.; Deng, Z.; Shi, F.; Zhou, Z.; Jiang, C.; Xu, Z.; Cui, X.; Li, W.; Jing, Y.; Han, B. Rebamipide-loaded chitosan nanoparticles accelerate prostatic wound healing by inhibiting M1 macrophage-mediated inflammation via the NF-κB signaling pathway. Biomater. Sci. 2020, 8, 912–925. [Google Scholar] [CrossRef]
- Hu, K.; Xiang, L.; Chen, J.; Qu, H.; Wan, Y.; Xiang, D. PLGA-liposome electrospun fiber delivery of miR-145 and PDGF-BB synergistically promoted wound healing. Chem. Eng. J. 2021, 422, 129951. [Google Scholar] [CrossRef]
- Devi, M.V.; Sobhana, S.L.; Shiny, P.J.; Ramanathan, G.; Felciya, S.J.G.; Poornima, V.; Thennarasu, S.; Fardim, P.; Sivagnanam, U.T. Durable nanofibrous matrices augmented with hydrotalcite-like compounds for cutaneous regeneration of burn wounds. Appl. Clay Sci. 2020, 187, 105476. [Google Scholar] [CrossRef]
- Ma, Y.; Duan, L.; Sun, J.; Gou, S.; Chen, F.; Liang, Y.; Dai, F.; Xiao, B. Oral nanotherapeutics based on Antheraea pernyi silk fibroin for synergistic treatment of ulcerative colitis. Biomaterials 2022, 282, 121410. [Google Scholar] [CrossRef]
- Ren, X.; Han, Y.; Wang, J.; Jiang, Y.; Yi, Z.; Xu, H.; Ke, Q. An aligned porous electrospun fibrous membrane with controlled drug delivery—An efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018, 70, 140–153. [Google Scholar] [CrossRef]
- Tang, T.; Jiang, H.; Yu, Y.; He, F.; Ji, S.; Liu, Y.; Wang, Z.; Xiao, S.; Tang, C.; Wang, G.-Y.; et al. A new method of wound treatment: Targeted therapy of skin wounds with reactive oxygen species-responsive nanoparticles containing SDF-1α. Int. J. Nanomed. 2015, 10, 6571–6585. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.U.; Lee, S.W.; Pangeni, R.; Byun, Y.; Yoon, I.-S.; Park, J.W. Preparation and in vivo evaluation of cationic elastic liposomes comprising highly skin-permeable growth factors combined with hyaluronic acid for enhanced diabetic wound-healing therapy. Acta Biomater. 2017, 57, 197–215. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Tashkhourian, J.; Ana, S.F.N. Topical delivery of chitosan-capped silver nanoparticles speeds up healing in burn wounds: A preclinical study. Carbohydr. Polym. 2018, 200, 82–92. [Google Scholar] [CrossRef]
- Salvo, J.; Sandoval, C. Role of copper nanoparticles in wound healing for chronic wounds: Literature review. Burn. Trauma 2022, 10, tkab047. [Google Scholar] [CrossRef]
- Rodríguez-Acosta, H.; Rivera, J.M.T.; Guerrero-Guzmán, A.; Hernández-Elizarraráz, E.; Díaz, J.A.H.; García, J.J.G.; Ramírez, P.E.P.; Ramírez, S.F.V.; Anguiano, A.C.R.; Juárez, G.V.; et al. Chronic wound healing by controlled release of chitosan hydrogels loaded with silver nanoparticles and calendula extract. J. Tissue Viabil. 2021, 31, 173–179. [Google Scholar] [CrossRef]
- Perez-Amodio, S.; Rubio, N.; Vila, O.F.; Navarro-Requena, C.; Castaño, O.; Sanchez-Ferrero, A.; Marti-Munoz, J.; Alsina-Giber, M.; Blanco, J.; Engel, E. Polymeric Composite Dressings Containing Calcium-Releasing Nanoparticles Accelerate Wound Healing in Diabetic Mice. Adv. Wound Care 2021, 10, 301–316. [Google Scholar] [CrossRef]
- Muhamed, I.; Sproul, E.P.; Ligler, F.S.; Brown, A.C. Fibrin Nanoparticles Coupled with Keratinocyte Growth Factor Enhance the Dermal Wound-Healing Rate. ACS Appl. Mater. Interfaces 2019, 11, 3771–3780. [Google Scholar] [CrossRef]
- Jirofti, N.; Golandi, M.; Movaffagh, J.; Ahmadi, F.S.; Kalalinia, F. Improvement of the Wound-Healing Process by Curcumin-Loaded Chitosan/Collagen Blend Electrospun Nanofibers: In Vitro and In Vivo Studies. ACS Biomater. Sci. Eng. 2021, 7, 3886–3897. [Google Scholar] [CrossRef]
- Schepler, H.; Neufurth, M.; Wang, S.; She, Z.; Schröder, H.C.; Wang, X.; Müller, W.E. Acceleration of chronic wound healing by bio-inorganic polyphosphate: In vitro studies and first clinical applications. Theranostics 2022, 12, 18–34. [Google Scholar] [CrossRef]
- Dickinson, L.E.; Gerecht, S. Engineered biopolymeric scaffolds for chronic wound healing. Front. Physiol. 2016, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Duscher, D.; Barrera, J.; Wong, V.W.; Maan, Z.N.; Whittam, A.J.; Januszyk, M.; Gurtner, G.C. Stem Cells in Wound Healing: The Future of Regenerative Medicine? A Mini-Review. Gerontology 2015, 62, 216–225. [Google Scholar] [CrossRef]
- Chen, M.; Przyborowski, M.; Berthiaume, F. Stem Cells for Skin Tissue Engineering and Wound Healing. Crit. Rev. Biomed. Eng. 2009, 37, 399–421. [Google Scholar] [CrossRef] [Green Version]
- Kølle, S.-F.T.; Fischer-Nielsen, A.; Mathiasen, A.B.; Elberg, J.J.; Oliveri, R.S.; Glovinski, P.V.; Kastrup, J.; Kirchhoff, M.; Rasmussen, B.S.; Talman, M.-L.M.; et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet 2013, 382, 1113–1120. [Google Scholar] [CrossRef]
- Tsai, H.-W.; Wang, P.-H.; Tsui, K.-H. Mesenchymal stem cell in wound healing and regeneration. J. Chin. Med. Assoc. 2018, 81, 223–224. [Google Scholar] [CrossRef]
- Hu, M.S.; Borrelli, M.R.; Lorenz, H.P.; Longaker, M.T.; Wan, D.C. Mesenchymal Stromal Cells and Cutaneous Wound Healing: A Comprehensive Review of the Background, Role, and Therapeutic Potential. Stem Cells Int. 2018, 2018, 6901983. [Google Scholar] [CrossRef] [Green Version]
- Dash, B.C.; Xu, Z.; Lin, L.; Koo, A.; Ndon, S.U.; Berthiaume, F.; Dardik, A.; Hsia, H.C. Stem Cells and Engineered Scaffolds for Regenerative Wound Healing. Bioengineering 2018, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Wang, M.-Y.; Tai, H.-C.; Cheng, N.-C. Cell sheet composed of adipose-derived stem cells demonstrates enhanced skin wound healing with reduced scar formation. Acta Biomater. 2018, 77, 191–200. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [Green Version]
- Rasulov, M.F.; Vasil’Chenkov, A.V.; Onishchenko, N.A.; Krasheninnikov, M.E.; Kravchenko, V.I.; Gorshenin, T.L.; Pidtsan, R.E.; Potapov, I.V. First experience in the use of bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull. Exp. Biol. Med. 2005, 139, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, S.; Fu, X. Autologous transplantation of bone marrow-derived mesenchymal stem cells: A promising therapeutic strategy for prevention of skin-graft contraction. Clin. Exp. Dermatol. 2012, 37, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Ján, V.; Ľuboš, D.; Miroslav, K.; Dušan, B.; Ľubomír, J.; Marcela, U.; Milan, B. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuroendocrinol. Lett. 2006, 27, 2. [Google Scholar]
- Aboulhoda, B.E.; Abd el Fattah, S. Bone marrow-derived versus adipose-derived stem cells in wound healing: Value and route of administration. Cell Tissue Res. 2018, 374, 285–302. [Google Scholar] [CrossRef]
- Abolgheit, S.; Abdelkader, S.; Aboushelib, M.; Omar, E.; Mehanna, R. Bone marrow-derived mesenchymal stem cells and extracellular vesicles enriched collagen chitosan scaffold in skin wound healing (a rat model). J. Biomater. Appl. 2020, 36, 128–139. [Google Scholar] [CrossRef]
- Luna, G.F.; Oehlmeyer, T.; Brandão, G.; Brassolatti, P.; Tosta, J.; Goto, L.; de Avó, L.; Leal, A.D.O. Use of human bone marrow mesenchymal stem cells immortalized by the expression of telomerase in wound healing in diabetic rats. Braz. J. Med. Biol. Res. 2021, 54. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Peng, Y.; Zhao, Y.; Qin, Y.; Zhang, Y.; Xiao, Z. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J. Nanobiotechnol. 2021, 19, 202. [Google Scholar] [CrossRef]
- Roshangar, L.; Rad, J.S.; Kheirjou, R.; Khosroshahi, A.F. Using 3D-bioprinting scaffold loaded with adipose-derived stem cells to burns wound healing. J. Tissue Eng. Regen. Med. 2021, 15, 546–555. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, B.; Shu, J.; Wang, H.; Han, Y.; Zeng, Q.; Chen, Y.; Xi, J.; Tao, R.; Pei, X.; et al. Human decellularized adipose matrix derived hydrogel assists mesenchymal stem cells delivery and accelerates chronic wound healing. J. Biomed. Mater. Res. Part A 2020, 109, 1418–1428. [Google Scholar] [CrossRef]
- Heidari, F.; Yari, A.; Rasoolijazi, H.; Soleimani, M.; Dehpoor, A.; Sajedi, N.; Veijouye, S.J.; Nobakht, M. Bulge Hair Follicle Stem Cells Accelerate Cutaneous Wound Healing in Rats. Wounds Compend. Clin. Res. Pract. 2016, 28, 132–141. [Google Scholar]
- Martínez, M.-L.; Escario, E.; Poblet, E.; Sánchez, D.; Buchón, F.-F.; Izeta, A.; Jimenez, F. Hair follicle–containing punch grafts accelerate chronic ulcer healing: A randomized controlled trial. J. Am. Acad. Dermatol. 2016, 75, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Li, M.; Li, X.; Zheng, Y.; Zhang, K.; Liu, X.; Cai, B.; Yin, G. PlncRNA-1 stimulates hair follicle stem cell differentiation in wound healing via the EZH2/ZEB1/MAPK1 axis. J. Gene Med. 2022, e3408. [Google Scholar] [CrossRef] [PubMed]
- Clayton, Z.E.; Tan, R.P.; Miravet, M.M.; Lennartsson, K.; Cooke, J.P.; Bursill, C.A.; Wise, S.G.; Patel, S. Induced pluripotent stem cell-derived endothelial cells promote angiogenesis and accelerate wound closure in a murine excisional wound healing model. Biosci. Rep. 2018, 38, BSR20180563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Ebisawa, K.; Kambe, M.; Kasai, T.; Suga, H.; Nakamura, K.; Narita, Y.; Ogata, A.; Kamei, Y. <Editors’ Choice> Effects of exosomes derived from the induced pluripotent stem cells on skin wound healing. Nagoya J. Med. Sci. 2018, 80, 141–153. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, R.; Bo, Y.; Zhang, M.; Chen, Y.; Wang, X.; Huang, M.; Liu, B.; Zhang, L. Induced pluripotent stem cells-derived microvesicles accelerate deep second-degree burn wound healing in mice through miR-16-5p-mediated promotion of keratinocytes migration. Theranostics 2020, 10, 9970–9983. [Google Scholar] [CrossRef]
- An, Y.; Liu, W.J.; Xue, P.; Ma, Y.; Zhang, L.; Zhu, B.; Qi, M.; Li, L.Y.; Zhang, Y.J.; Wang, Q.T.; et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death Dis. 2018, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Choi, E.; Cha, M.-J.; Hwang, K.-C. Cell Adhesion and Long-Term Survival of Transplanted Mesenchymal Stem Cells: A Prerequisite for Cell Therapy. Oxidative Med. Cell. Longev. 2015, 2015, 632902. [Google Scholar] [CrossRef] [Green Version]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Central Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Navone, S.E.; Pascucci, L.; Dossena, M.; Ferri, A.; Invernici, G.; Acerbi, F.; Cristini, S.; Bedini, G.; Tosetti, V.; Ceserani, V.; et al. Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Res. Ther. 2014, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Tuca, A.-C.; Ertl, J.; Hingerl, K.; Pichlsberger, M.; Fuchs, J.; Wurzer, P.; Pfeiffer, D.; Bubalo, V.; Parvizi, D.; Kamolz, L.-P.; et al. Comparison of Matrigel and Matriderm as a carrier for human amnion-derived mesenchymal stem cells in wound healing. Placenta 2016, 48, 99–103. [Google Scholar] [CrossRef]
- Goodarzi, P.; Larijani, B.; Alavi-Moghadam, S.; Tayanloo-Beik, A.; Mohamadi-Jahani, F.; Ranjbaran, N.; Payab, M.; Falahzadeh, K.; Mousavi, M.; Arjmand, B. Mesenchymal Stem Cells-Derived Exosomes for Wound Regeneration. Cell Biol. Transl. Med. 2018, 1119, 119–131. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Lei, X.; Tan, J.; Xie, H. Mesenchymal stem cell-based therapy for burn wound healing. Burn. Trauma 2021, 9, tkab002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cao, Y.; Li, X.; Hu, L.; Taieb, S.K.; Zhu, X.; Zhang, J.; Feng, Y.; Zhao, R.; Wang, M.; et al. Cd271 mediates proliferation and differentiation of epidermal stem cells to support cutaneous burn wound healing. Cell Tissue Res. 2017, 371, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zheng, J.; Li, Z.; Liu, M. Halloysite nanotubes coated 3D printed PLA pattern for guiding human mesenchymal stem cells (hMSCs) orientation. Chem. Eng. J. 2018, 359, 672–683. [Google Scholar] [CrossRef]
- Rashad, A.; Mohamed-Ahmed, S.; Ojansivu, M.; Berstad, K.; Yassin, M.A.; Kivijärvi, T.; Heggset, E.B.; Syverud, K.; Mustafa, K. Coating 3D Printed Polycaprolactone Scaffolds with Nanocellulose Promotes Growth and Differentiation of Mesenchymal Stem Cells. Biomacromolecules 2018, 19, 4307–4319. [Google Scholar] [CrossRef]
- Sun, L.; Xu, R.; Sun, X.; Duan, Y.; Han, Y.; Zhao, Y.; Qian, H.; Zhu, W.; Xu, W. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy 2016, 18, 413–422. [Google Scholar] [CrossRef]
- Kocan, B.; Maziarz, A.; Tabarkiewicz, J.; Ochiya, T.; Banaś-Ząbczyk, A. Trophic Activity and Phenotype of Adipose Tissue-Derived Mesenchymal Stem Cells as a Background of Their Regenerative Potential. Stem Cells Int. 2017, 2017, 13. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.S.; Kim, S.; Yang, C.E.; Choi, Y.; Song, S.Y.; Kim, H.O. Human Adipose Mesenchymal Stem Cell-Derived Exosomes: A Key Player in Wound Healing. Tissue Eng. Regen. Med. 2021, 18, 537–548. [Google Scholar] [CrossRef]
- Weiliang, Z.; Lili, G. Research advances in the application of adipose-derived stem cells derived exosomes in cutaneous wound healing. Ann. Dermatol. 2021, 33, 309. [Google Scholar] [CrossRef]
- Jung, J.-A.; Yoon, Y.-D.; Lee, H.-W.; Kang, S.-R.; Han, S.-K. Comparison of human umbilical cord blood-derived mesenchymal stem cells with healthy fibroblasts on wound-healing activity of diabetic fibroblasts. Int. Wound J. 2017, 15, 133–139. [Google Scholar] [CrossRef]
- Shrestha, C.; Zhao, L.; Chen, K.; He, H.; Mo, Z. Enhanced Healing of Diabetic Wounds by Subcutaneous Administration of Human Umbilical Cord Derived Stem Cells and Their Conditioned Media. Int. J. Endocrinol. 2013, 2013, 592454. [Google Scholar] [CrossRef] [PubMed]
- Afzali, L.; Mirahmadi-Babaheydari, F.; Shojaei-Ghahrizjani, F.; Rahmati, S.; Shahmoradi, B.; Banitalebi-Dehkordi, M. The Effect of Encapsulated Umbilical Cord-derived Mesenchymal Stem Cells in PRPCryogel on Regeneration of Grade-II Burn Wounds. Regen. Eng. Transl. Med. 2020, 8, 75–85. [Google Scholar] [CrossRef]
- Nazempour, M.; Mehrabani, D.; Mehdinavaz-Aghdam, R.; Hashemi, S.S.; Derakhshanfar, A.; Zare, S.; Zardosht, M.; Moayedi, J.; Vahedi, M. The effect of allogenic human Wharton’s jelly stem cells seeded onto acellular dermal matrix in healing of rat burn wounds. J. Cosmet. Dermatol. 2020, 19, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Guasch, G. The epithelial stem cell niche in skin. In Biology and Engineering of Stem Cell Niches; Elsevier: Amsterdam, The Netherlands, 2017; pp. 127–143. [Google Scholar]
- Wang, L.; Su, Y.; Huang, C.; Yin, Y.; Chu, A.; Knupp, A.; Tang, Y. NANOG and LIN28 dramatically improve human cell reprogramming by modulating LIN41 and canonical WNT activities. Biol. Open 2019, 8, bio047225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka, S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell 2007, 1, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Bilousova, G.; Chen, J.; Roop, D.R. Differentiation of Mouse Induced Pluripotent Stem Cells into a Multipotent Keratinocyte Lineage. J. Investig. Dermatol. 2011, 131, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Zheng, Y.; Burrows, M.; Liu, S.; Wei, Z.; Nace, A.; Guo, W.; Kumar, S.; Cotsarelis, G.; Xu, X. Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells. Nat. Commun. 2014, 5, 3071. [Google Scholar] [CrossRef] [Green Version]
- Itoh, M.; Umegaki-Arao, N.; Guo, Z.; Liu, L.; Higgins, C.A.; Christiano, A.M. Generation of 3D Skin Equivalents Fully Reconstituted from Human Induced Pluripotent Stem Cells (iPSCs). PLoS ONE 2013, 8, e77673. [Google Scholar] [CrossRef] [Green Version]
- Umegaki-Arao, N.; Pasmooij, A.M.G.; Itoh, M.; Cerise, J.E.; Guo, Z.; Levy, B.; Gostyński, A.; Rothman, L.R.; Jonkman, M.F.; Christiano, A.M. Induced pluripotent stem cells from human revertant keratinocytes for the treatment of epidermolysis bullosa. Sci. Transl. Med. 2014, 6, 264ra164. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. 3D bioprinting applications for the printing of skin: A brief study. Sens. Int. 2021, 2, 100123. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Dufort, E.M.; Udo, T.; Wilberschied, L.A.; Kumar, J.; Tesoriero, J.; Weinberg, P.; Kirkwood, J.; Muse, A.; DeHovitz, J.; et al. Association of Treatment with Hydroxychloroquine or Azithromycin with In-Hospital Mortality in Patients with COVID-19 in New York State. JAMA 2020, 323, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhao, J.; Zhang, J.; Li, B.; Gou, Z.; Gou, M.; Li, X. Bioprinting of skin constructs for wound healing. Burn. Trauma 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, W.L.; Wang, S.; Yeong, W.Y.; Naing, M.W. Skin Bioprinting: Impending Reality or Fantasy? Trends Biotechnol. 2016, 34, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.; Nandakumar, K.; Sabu, T. Microbial barrier property and blood compatibility studies of electrospun Poly-ƹ-caprolactone/zinc oxide nanocomposite scaffolds. Журнал Сибирскoгo федеральнoгo университета. Биoлoгия 2017, 10, 226–236. [Google Scholar]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Fisch, P.; Holub, M.; Zenobi-Wong, M. Improved accuracy and precision of bioprinting through progressive cavity pump-controlled extrusion. Biofabrication 2020, 13, 015012. [Google Scholar] [CrossRef]
- VijayaVenkataRaman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef]
- Ishack, S.; Lipner, S.R. A Review of 3-Dimensional Skin Bioprinting Techniques: Applications, Approaches, and Trends. Dermatol. Surg. 2020, 46, 1500–1505. [Google Scholar] [CrossRef]
- Perez-Valle, A.; Del Amo, C.; Andia, I. Overview of Current Advances in Extrusion Bioprinting for Skin Applications. Int. J. Mol. Sci. 2020, 21, 6679. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Ning, L.; Chen, X. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol. J. 2017, 12, 1600671. [Google Scholar] [CrossRef] [PubMed]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Skylar-Scott, M.A.; Mueller, J.; Visser, C.W.; Lewis, J.A. Voxelated soft matter via multimaterial multinozzle 3D printing. Nature 2019, 575, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shusteff, M.; Spadaccini, C.M.; Taylor, H.K. Volumetric additive manufacturing via tomographic reconstruction. Science 2019, 363, 1075–1079. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J.-S.; Gao, G.; Cho, D.-W. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017, 9, 025034. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [Green Version]
- Groll, J.; Burdick, J.A.; Cho, D.-W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2019, 11, 013001. [Google Scholar] [CrossRef]
- Donderwinkel, I.; van Hest, J.C.M.; Cameron, N.R. Bio-inks for 3D bioprinting: Recent advances and future prospects. Polym. Chem. 2017, 8, 4451–4471. [Google Scholar] [CrossRef] [Green Version]
- Masri, S.; Fauzi, M. Current Insight of Printability Quality Improvement Strategies in Natural-Based Bioinks for Skin Regeneration and Wound Healing. Polymers 2021, 13, 1011. [Google Scholar] [CrossRef]
- Masri, S.; Zawani, M.; Zulkiflee, I.; Salleh, A.; Fadilah, N.I.; Maarof, M.; Wen, A.P.Y.; Duman, F.; Tabata, Y.; Aziz, I.A.; et al. Cellular Interaction of Human Skin Cells towards Natural Bioink via 3D-Bioprinting Technologies for Chronic Wound: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 476. [Google Scholar] [CrossRef]
- Hazur, J.; Endrizzi, N.; Schubert, D.W.; Boccaccini, A.R.; Fabry, B. Stress relaxation amplitude of hydrogels determines migration, proliferation, and morphology of cells in 3-D culture. Biomater. Sci. 2021, 10, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Velasco, D.; Quílez, C.; Garcia, M.; Del Cañizo, J.F.; Jorcano, J.L. 3D human skin bioprinting: A view from the bio side. J. 3D Print. Med. 2018, 2, 141–162. [Google Scholar] [CrossRef]
- Lee, W.; Debasitis, J.C.; Lee, V.K.; Lee, J.-H.; Fischer, K.; Edminster, K.; Park, J.-K.; Yoo, S.-S. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009, 30, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Kuhn, S.; Sorg, H.; Gruene, M.; Schlie, S.; Gaebel, R.; Polchow, B.; Reimers, K.; Stoelting, S.; Ma, N.; et al. Laser Printing of Skin Cells and Human Stem Cells. Tissue Eng. Part C Methods 2010, 16, 847–854. [Google Scholar] [CrossRef]
- Binder, K.W.; Zhao, W.; Aboushwareb, T.; Dice, D.; Atala, A.; Yoo, J.J. In situ bioprinting of the skin for burns. J. Am. Coll. Surg. 2010, 211, S76. [Google Scholar] [CrossRef]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef] [Green Version]
- Hakimi, N.; Cheng, R.; Leng, L.; Sotoudehfar, M.; Ba, P.Q.; Bakhtyar, N.; Amini-Nik, S.; Jeschke, M.G.; Günther, A. Handheld skin printer: In situ formation of planar biomaterials and tissues. Lab Chip 2018, 18, 1440–1451. [Google Scholar] [CrossRef]
- Admane, P.; Gupta, A.C.; Jois, P.; Roy, S.; Lakshmanan, C.C.; Kalsi, G.; Bandyopadhyay, B.; Ghosh, S. Direct 3D bioprinted full-thickness skin constructs recapitulate regulatory signaling pathways and physiology of human skin. Bioprinting 2019, 15, e00051. [Google Scholar] [CrossRef]
- Derr, K.; Zou, J.; Luo, K.; Song, M.J.; Sittampalam, G.S.; Zhou, C.; Michael, S.; Ferrer, M.; Derr, P. Fully Three-Dimensional Bioprinted Skin Equivalent Constructs with Validated Morphology and Barrier Function. Tissue Eng. Part C Methods 2019, 25, 334–343. [Google Scholar] [CrossRef]
- Liu, X.; Michael, S.; Bharti, K.; Ferrer, M.; Song, M.J. A biofabricated vascularized skin model of atopic dermatitis for preclinical studies. Biofabrication 2020, 12, 035002. [Google Scholar] [CrossRef]
- Zidarič, T.; Milojević, M.; Gradišnik, L.; Kleinschek, K.S.; Maver, U.; Maver, T. Polysaccharide-Based Bioink Formulation for 3D Bioprinting of an In Vitro Model of the Human Dermis. Nanomaterials 2020, 10, 733. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Kwon, Y.W.; Kong, J.-S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.-B.; Lee, H.; Kim, J.H.; Cho, D.-W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells. Tissue Eng. Part A 2020, 26, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.M.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J.; et al. Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds. Tissue Eng. Part A 2020, 26, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D.-W. 3D Cell Printing of Perfusable Vascularized Human Skin Equivalent Composed of Epidermis, Dermis, and Hypodermis for Better Structural Recapitulation of Native Skin. Adv. Healthc. Mater. 2019, 8, 1801019. [Google Scholar] [CrossRef]
- Lian, Q.; Jiao, T.; Zhao, T.; Wang, H.; Yang, S.; Li, D. 3D Bioprinted Skin Substitutes for Accelerated Wound Healing and Reduced Scar. J. Bionic Eng. 2021, 18, 900–914. [Google Scholar] [CrossRef]
- Jara, C.P.; Catarino, C.M.; Lei, Y.; Velloso, L.A.; Karande, P.; Velander, W.H.; de Araujo, E.P. Demonstration of re-epithelialization in a bioprinted human skin equivalent wound model. Bioprinting 2020, 24, e00102. [Google Scholar] [CrossRef]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Yu, Y.; Zhao, Y. In Situ 3D Bioprinting Living Photosynthetic Scaffolds for Autotrophic Wound Healing. Research 2022, 2022, 11. [Google Scholar] [CrossRef]
- Niu, C.; Wang, L.; Ji, D.; Ren, M.; Ke, D.; Fu, Q.; Zhang, K.; Yang, X. Fabrication of SA/Gel/C scaffold with 3D bioprinting to generate micro-nano porosity structure for skin wound healing: A detailed animal in vivo study. Cell Regen. 2022, 11, 10. [Google Scholar] [CrossRef]
- Ma, J.; Qin, C.; Wu, J.; Zhang, H.; Zhuang, H.; Zhang, M.; Zhang, Z.; Ma, L.; Wang, X.; Ma, B.; et al. 3D Printing of Strontium Silicate Microcylinder-Containing Multicellular Biomaterial Inks for Vascularized Skin Regeneration. Adv. Heal. Mater. 2021, 10, 2100523. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.J.; Bae, S.; Jung, Y.-S.; Kim, J.C.; Park, S.K.; Suh, J.S.; Yi, S.J.; Ahn, S.H.; Lim, J.O. Enhanced wound healing using a 3D printed VEGF-mimicking peptide incorporated hydrogel patch in a pig model. Biomed. Mater. 2021, 16, 045013. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; He, J.-M.; Wang, Z.; Liu, L.-L.; Li, X.-L.; Wu, C.-X.; Chen, C.-S.; Tu, M. Proangiogenic peptide nanofiber hydrogel/3D printed scaffold for dermal regeneration. Chem. Eng. J. 2020, 424, 128146. [Google Scholar] [CrossRef]
- Guan, G.; Lv, Q.; Liu, S.; Jiang, Z.; Zhou, C.; Liao, W. 3D-bioprinted peptide coupling patches for wound healing. Mater. Today Bio 2021, 13, 100188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2021, 10, 15–31. [Google Scholar] [CrossRef]
- Sutcliffe, J.E.; Thrasivoulou, C.; E Serena, T.; Madden, L.; Richards, T.; Phillips, A.R.; Becker, D.L. Changes in the extracellular matrix surrounding human chronic wounds revealed by 2-photon imaging. Int. Wound J. 2017, 14, 1225–1236. [Google Scholar] [CrossRef]
- Kusindarta, D.L.; Wihadmadyatami, H. The role of extracellular matrix in tissue regeneration. Tissue Regen. 2018, 65. [Google Scholar] [CrossRef] [Green Version]
- Assunção, M.; Dehghan-Baniani, D.; Yiu, C.H.K.; Später, T.; Beyer, S.; Blocki, A. Cell-Derived Extracellular Matrix for Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 602009. [Google Scholar] [CrossRef]
- Turner, N. Special Issue: Application of Extracellular Matrix in Regenerative Medicine. Appl. Sci. 2021, 11, 3262. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Rasouli, M.; Rahimi, A.; Soleimani, M.; Keshel, S.H. The interplay between extracellular matrix and progenitor/stem cells during wound healing: Opportunities and future directions. Acta Histochem. 2021, 123, 151785. [Google Scholar] [CrossRef] [PubMed]
- Djavid, G.E.; Tabaie, S.M.; Tajali, S.B.; Totounchi, M.; Farhoud, A.; Fateh, M.; Ghafghazi, M.; Koosha, M.; Taghizadeh, S. Application of a collagen matrix dressing on a neuropathic diabetic foot ulcer: A randomised control trial. J. Wound Care 2020, 29, S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Z.; Zhao, Y.; Feng, Y.; Zvyagin, A.V.; Wang, J.; Yang, X.; Yang, B.; Lin, Q. Novel Diabetic Foot Wound Dressing Based on Multifunctional Hydrogels with Extensive Temperature-Tolerant, Durable, Adhesive, and Intrinsic Antibacterial Properties. ACS Appl. Mater. Interfaces 2021, 13, 26770–26781. [Google Scholar] [CrossRef]
- Cramer, M.C.; Badylak, S.F. Extracellular matrix-based biomaterials and their influence upon cell behavior. Ann. Biomed. Eng. 2020, 48, 2132–2153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, R.; Han, C.; Huang, L. Extracellular matrix grafts: From preparation to application (Review). Int. J. Mol. Med. 2020, 47, 463–474. [Google Scholar] [CrossRef]
- Zhang, Q.; Johnson, J.A.; Dunne, L.W.; Chen, Y.; Iyyanki, T.; Wu, Y.; Chang, E.I.; Branch-Brooks, C.D.; Robb, G.L.; Butler, C.E. Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater. 2016, 35, 166–184. [Google Scholar] [CrossRef] [Green Version]
- Milan, P.B.; Pazouki, A.; Joghataei, M.T.; Mozafari, M.; Amini, N.; Kargozar, S.; Amoupour, M.; Latifi, N.; Samadikuchaksaraei, A. Decellularization and preservation of human skin: A platform for tissue engineering and reconstructive surgery. Methods 2020, 171, 62–67. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Li, Y.; Liu, S.; Long, K.; Zhao, Q.; Zhang, Y.; Deng, Z.; Jin, Y. Functional Neovascularization in Tissue Engineering with Porcine Acellular Dermal Matrix and Human Umbilical Vein Endothelial Cells. Tissue Eng. Part C Methods 2011, 17, 423–433. [Google Scholar] [CrossRef]
- Takami, Y.; Yamaguchi, R.; Ono, S.; Hyakusoku, H. Clinical Application and Histological Properties of Autologous Tissue-engineered Skin Equivalents Using an Acellular Dermal Matrix. J. Nippon Med. Sch. 2014, 81, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Sotnichenko, A.; Gilevich, I.; Melkonyan, K. Development of methods for obtaining dermal extracellular matrix. Vestnik Transplantologii i Iskusstvennykh Organov 2020, 21, 81–87. [Google Scholar]
- Choi, J.S.; Kim, J.D.; Yoon, H.S.; Cho, Y.W. Full-Thickness Skin Wound Healing Using Human Placenta-Derived Extracellular Matrix Containing Bioactive Molecules. Tissue Eng. Part A 2013, 19, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milan, P.B.; Lotfibakhshaiesh, N.; Joghataie, M.; Ai, J.; Pazouki, A.; Kaplan, D.; Kargozar, S.; Amini, N.; Hamblin, M.; Mozafari, M.; et al. Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells. Acta Biomater. 2016, 45, 234–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groeber, F.; Engelhardt, L.; Lange, J.; Kurdyn, S.; Schmid, F.F.; Rücker, C.; Mielke, S.; Walles, H.; Hansmann, J. A first vascularized skin equivalent for as an alternative to animal experimentation. ALTEX 2016, 33, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Hoganson, D.; O’Doherty, E.M.; Owens, G.E.; Harilal, D.O.; Goldman, S.M.; Bowley, C.M.; Neville, C.M.; Kronengold, R.T.; Vacanti, J.P. The retention of extracellular matrix proteins and angiogenic and mitogenic cytokines in a decellularized porcine dermis. Biomaterials 2010, 31, 6730–6737. [Google Scholar] [CrossRef]
- Parmaksiz, M.; Dogan, A.; Odabas, S.; Elçin, A.E.; Elçin, Y.M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 2016, 11, 022003. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zheng, J.; Wang, Y.; Zhang, W.; Hu, D. Emerging progress on the mechanism and technology in wound repair. Biomed. Pharmacother. 2019, 117, 109191. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.; Farouk, N.; Dahy, A.A.; Abu-Elsoud, A.; Khattab, R.F.; Mohammed, S.E.; Gad, L.A.; Altramsy, A.; Hussein, E.; Farahat, A. Objective assessment of Platelet-Rich Plasma (PRP) potentiality in the treatment of Chronic leg Ulcer: RCT on 80 patients with Venous ulcer. J. Cosmet. Dermatol. 2021, 20, 3257–3263. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Z.; Hunt, C.; Morrow, A.S.; Urtecho, M.; Amin, M.; Shah, S.; Hasan, B.; Abd-Rabu, R.; Ashmore, Z. The Effectiveness and Safety of Platelet-Rich Plasma for Chronic Wounds: A Systematic Review and Meta-analysis. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2021; pp. 2407–2417. [Google Scholar]
- Ahmad, Z.; Howard, D.; A Brooks, R.; Wardale, J.; Henson, F.; Getgood, A.; Rushton, N. The role of platelet rich plasma in musculoskeletal science. JRSM Short Rep. 2012, 3, 1–9. [Google Scholar] [CrossRef]
- Jinming, W.; Caiyue, L.; Baojin, W.; Antang, L.; Yingfan, Z.; Hui, W.; Lie, Z.; Hua, J. Effects of Platelet-Rich Plasma on Tissue Expansion in Rabbits. Aesthetic Plast. Surg. 2017, 41, 454–460. [Google Scholar] [CrossRef]
- Chicharro-Alcántara, D.; Rubio-Zaragoza, M.; Damiá-Giménez, E.; Carrillo-Poveda, J.M.; Cuervo-Serrato, B.; Peláez-Gorrea, P.; Sopena-Juncosa, J.J. Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management. J. Funct. Biomater. 2018, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Gonchar, I.; Lipunov, A.; Afanasov, I.; Larina, V.; Faller, A.; Kibardin, A. Platelet rich plasma and growth factors cocktails for diabetic foot ulcers treatment: State of art developments and future prospects. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Griffeth, R.J.; García-Párraga, D.; Mellado-López, M.; Crespo-Picazo, J.L.; Soriano-Navarro, M.; Martínez-Romero, A.; Moreno-Manzano, V. Platelet-Rich Plasma and Adipose-Derived Mesenchymal Stem Cells for Regenerative Medicine-Associated Treatments in Bottlenose Dolphins (Tursiops truncatus). PLoS ONE 2014, 9, e108439. [Google Scholar] [CrossRef] [PubMed]
- Suthar, M.; Gupta, S.; Bukhari, S.; Ponemone, V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: A case series. J. Biomed. Sci. 2017, 24, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dionyssiou, D.; Demiri, E.; Foroglou, P.; Cheva, A.; Saratzis, N.; Aivazidis, C.; Karkavelas, G. The effectiveness of intralesional injection of platelet-rich plasma in accelerating the healing of chronic ulcers: An experimental and clinical study. Int. Wound J. 2012, 10, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.A.; Jolly, D.G.; Worden, C.E., Sr.; Hendren, D.G.; Kane, C. Platelet-rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp. Mol. Pathol. 2003, 74, 244–255. [Google Scholar] [CrossRef]
- Jee, C.-H.; Eom, N.-Y.; Jang, H.-M.; Jung, H.-W.; Choi, E.-S.; Won, J.-H.; Hong, I.-H.; Kang, B.-T.; Jeong, D.W.; Jung, D.-I. Effect of autologous platelet-rich plasma application on cutaneous wound healing in dogs. J. Veter Sci. 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Lee, H.-W.; Reddy, M.S.; Geurs, N.; Palcanis, K.G.; Lemons, J.E.; Rahemtulla, F.G.; Ho, K.-J.; Chen, D.-T.; Davis, C.R.; Feldman, D.S. Efficacy of Platelet-Rich Plasma on Wound Healing in Rabbits. J. Periodontol. 2008, 79, 691–696. [Google Scholar] [CrossRef]
- Xu, P.; Wu, Y.; Zhou, L.; Yang, Z.; Zhang, X.; Hu, X.; Yang, J.; Wang, M.; Wang, B.; Luo, G.; et al. Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burn. Trauma 2020, 8, tkaa028. [Google Scholar] [CrossRef]
- Knighton, D.R.; Ciresi, K.; Fiegel, V.D.; Schumerth, S.; Butler, E.; Cerra, F. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surg. Gynecol. Obstet. 1990, 170, 56–60. [Google Scholar]
- Ostvar, O.; Shadvar, S.; Yahaghi, E.; Azma, K.; Fayyaz, A.F.; Ahmadi, K.; Nowrouzian, I. RETRACTED ARTICLE: Effect of platelet-rich plasma on the healing of cutaneous defects exposed to acute to chronic wounds: A clinico-histopathologic study in rabbits. Diagn. Pathol. 2015, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Villela, D.L.; Santos, V.L.C. Evidence on the use of platelet-rich plasma for diabetic ulcer: A systematic review. Growth Factors 2010, 28, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Pallua, N.; Wolter, T.; Markowicz, M. Platelet-rich plasma in burns. Burns 2010, 36, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Sommeling, C.; Heyneman, A.; Hoeksema, H.; Verbelen, J.; Stillaert, F.; Monstrey, S. The use of platelet-rich plasma in plastic surgery: A systematic review. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 301–311. [Google Scholar] [CrossRef]
- Martinez-Zapata, M.J.; Martí-Carvajal, A.J.; Sola, I.; Expósito, J.A.; Bolibar, I.; Rodriguez, L.; Garcia, J.; Zaror, C. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst. Rev. 2016, 5. [Google Scholar] [CrossRef]
- Lacci, K.M.; Dardik, A. Platelet-rich plasma: Support for its use in wound healing. Yale J. Biol. Med. 2010, 83, 1–9. [Google Scholar] [PubMed]
- Roubelakis, M.G.; Trohatou, O.; Roubelakis, A.; Mili, E.; Kalaitzopoulos, I.; Papazoglou, G.; Pappa, Κ.I.; Anagnou, N.P. Reports. Platelet-rich plasma (PRP) promotes fetal mesenchymal stem/stromal cell migration and wound healing process. Stem Cell Rev. Rep. 2014, 10, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Babaei, V.; Afradi, H.; Gohardani, H.; Nasseri, F.; Azarafza, M.; Teimourian, S. Management of chronic diabetic foot ulcers using platelet-rich plasma. J. Wound Care 2017, 26, 784–787. [Google Scholar] [CrossRef]
- Popescu, M.N.; Iliescu, M.G.; Beiu, C.; Popa, L.G.; Mihai, M.M.; Berteanu, M.; Ionescu, A.M. Autologous Platelet-Rich Plasma Efficacy in the Field of Regenerative Medicine: Product and Quality Control. BioMed Res. Int. 2021, 2021, 4672959. [Google Scholar] [CrossRef]
- Emmert, S.; Pantermehl, S.; Foth, A.; Waletzko-Hellwig, J.; Hellwig, G.; Bader, R.; Illner, S.; Grabow, N.; Bekeschus, S.; Weltmann, K.-D.; et al. Combining Biocompatible and Biodegradable Scaffolds and Cold Atmospheric Plasma for Chronic Wound Regeneration. Int. J. Mol. Sci. 2021, 22, 9199. [Google Scholar] [CrossRef]
- Lu, X.; Laroussi, M.; Puech, V. On atmospheric-pressure non-equilibrium plasma jets and plasma bullets. Plasma Sources Sci. Technol. 2012, 21, 045006. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.-Y.; Lin, Z.-H.; Cheng, Y.-P.; Chiu, H.-Y.; Yeh, N.-L.; Wu, T.-K.; Wu, J.-S. Wound Healing in Streptozotocin-Induced Diabetic Rats Using Atmospheric-Pressure Argon Plasma Jet. Sci. Rep. 2018, 8, 12214. [Google Scholar] [CrossRef] [PubMed]
- Nasruddin; Nakajima, Y.; Mukai, K.; Rahayu, H.S.E.; Nur, M.; Ishijima, T.; Enomoto, H.; Uesugi, Y.; Sugama, J.; Nakatani, T. Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clin. Plasma Med. 2014, 2, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Darmawati, S.; Rohmani, A.; Nurani, L.H.; Prastiyanto, M.E.; Dewi, S.S.; Salsabila, N.; Wahyuningtyas, E.S.; Murdiya, F.; Sikumbang, I.M.; Rohmah, R.N.; et al. When plasma jet is effective for chronic wound bacteria inactivation, is it also effective for wound healing? Clin. Plasma Med. 2019, 14, 100085. [Google Scholar] [CrossRef]
- Martinez, L.G.; Dhruv, A.; Lin, L.; Balaras, E.; Keidar, M. Interaction between a helium atmospheric plasma jet and targets and dynamics of the interface. Plasma Sources Sci. Technol. 2019, 28, 115002. [Google Scholar] [CrossRef]
- Hiller, J.; Stratmann, B.; Timm, J.; Costea, T.; Tschoepe, D. Enhanced growth factor expression in chronic diabetic wounds treated by cold atmospheric plasma. Diabet. Med. 2022, 39, e14787. [Google Scholar] [CrossRef] [PubMed]
- Tiede, R.; Hirschberg, J.; Daeschlein, G.; von Woedtke, T.; Vioel, W.; Emmert, S. Plasma Applications: A Dermatological View. Contrib. Plasma Phys. 2014, 54, 118–130. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxidative Med. Cell. Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef] [Green Version]
- Isbary, G.; Stolz, W.; Shimizu, T.; Monetti, R.; Bunk, W.; Schmidt, H.-U.; Morfill, G.; Klämpfl, T.; Steffes, B.; Thomas, H.; et al. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin. Plasma Med. 2013, 1, 25–30. [Google Scholar] [CrossRef]
- Fathollah, S.; Mirpour, S.; Mansouri, P.; Dehpour, A.R.; Ghoranneviss, M.; Rahimi, N.; Naraghi, Z.S.; Chalangari, R.; Chalangari, K.M. Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Sci. Rep. 2016, 6, 19144. [Google Scholar] [CrossRef] [Green Version]
- Assadian, O.; Ousey, K.; Daeschlein, G.; Kramer, A.; Parker, C.; Tanner, J.; Leaper, D.J. Effects and safety of atmospheric low-temperature plasma on bacterial reduction in chronic wounds and wound size reduction: A systematic review and meta-analysis. Int. Wound J. 2018, 16, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuangsuwanich, A.; Assadamongkol, T.; Boonyawan, D. The healing effect of low-temperature atmospheric-pressure plasma in pressure ulcer: A randomized controlled trial. Int. J. Low. Extrem. Wounds 2016, 15, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.-F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold Atmospheric Plasma (CAP) Changes Gene Expression of Key Molecules of the Wound Healing Machinery and Improves Wound Healing In Vitro and In Vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisch, T.; Helmke, A.; Schleusser, S.; Song, J.; Liodaki, E.; Stang, F.H.; Mailaender, P.; Kraemer, R. Improvement of cutaneous microcirculation by cold atmospheric plasma (CAP): Results of a controlled, prospective cohort study. Microvasc. Res. 2015, 104, 55–62. [Google Scholar] [CrossRef]
- Isbary, G.; Morfill, G.; Schmidt, H.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.; Morfill, G.; Schmidt, H.-U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef]
- Heinlin, J.; Zimmermann, J.L.; Zeman, F.; Bunk, W.; Isbary, G.; Landthaler, M.; Maisch, T.; Monetti, R.; Morfill, G.; Shimizu, T.; et al. Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair Regen. 2013, 21, 800–807. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Xia, C.; Yang, X.; Cao, Z.; Zheng, L.; Ko, R.; Shen, C.; Yang, C.; Cheng, C. Cold atmospheric plasma promotes different types of superficial skin erosion wounds healing. Int. Wound J. 2019, 16, 1103–1111. [Google Scholar] [CrossRef]
- Brehmer, F.; Haenssle, H.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Görlitz, A.; Simon, D.; Schön, M.; Wandke, D.; Emmert, S.; et al. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm® VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT 01415622). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 148–155. [Google Scholar] [CrossRef]
- Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Czaika, V.; Richter, H.; Bob, A.; von Hutten, J.; Painsi, C.; Hüge, R.; et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J. Wound Care 2015, 24, 196–203. [Google Scholar] [CrossRef]
- Klebes, M.; Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Richter, H.; Bob, A.; von Hutten, J.; Krediet, J.T.; Kramer, A.; et al. Combined antibacterial effects of tissue-tolerable plasma and a modern conventional liquid antiseptic on chronic wound treatment. J. Biophoton. 2014, 8, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, B.; Costea, T.-C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W. Effect of cold atmospheric plasma therapy vs. standard therapy placebo on wound healing in patients with diabetic foot ulcers: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef] [PubMed]
- Lou, R.; Chen, J.; Zhou, F.; Wang, C.; Leung, C.-H.; Lin, L. Exosome-cargoed microRNAs: Potential therapeutic molecules for diabetic wound healing. Drug Discov. Today 2022. [Google Scholar] [CrossRef]
- Ashoori, M.R.; Rahmati-Yamchi, M.; Ostadrahimi, A.; Aval, S.F.; Zarghami, N. MicroRNAs and adipocytokines: Promising biomarkers for pharmacological targets in diabetes mellitus and its complications. Biomed. Pharmacother. 2017, 93, 1326–1336. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, J.; Chan, Y.C.; Sen, C.K. MicroRNAs in skin and wound healing. Physiol. Genom. 2011, 43, 543–556. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Ding, M.; Liu, D.-W.; Liu, Y.; Mao, Y.-G.; Peng, Y. MicroRNA profiling in cutaneous wounds of diabetic rats. Genet. Mol. Res. 2015, 14, 9614–9625. [Google Scholar] [CrossRef]
- Soliman, A.M.; Das, S.; Ghafar, N.A.; Teoh, S.L. Role of MicroRNA in Proliferation Phase of Wound Healing. Front. Genet. 2018, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Mulholland, E.J.; Dunne, N.; McCarthy, H.O. MicroRNA as Therapeutic Targets for Chronic Wound Healing. Mol. Ther. Nucleic Acids 2017, 8, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Xu, X.; Xiao, L.; Wang, L.; Qiang, S. The Role of microRNA in the Inflammatory Response of Wound Healing. Front. Immunol. 2022, 13, 1231. [Google Scholar] [CrossRef]
- Tanaka, K.; Kim, S.E.; Yano, H.; Matsumoto, G.; Ohuchida, R.; Ishikura, Y.; Araki, M.; Araki, K.; Park, S.; Komatsu, T.; et al. MiR-142 Is Required for Staphylococcus aureus Clearance at Skin Wound Sites via Small GTPase-Mediated Regulation of the Neutrophil Actin Cytoskeleton. J. Investig. Dermatol. 2017, 137, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Primo, M.; Bak, R.; Schibler, B.; Mikkelsen, J.G. Regulation of pro-inflammatory cytokines TNFα and IL24 by microRNA-203 in primary keratinocytes. Cytokine 2012, 60, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, X.; Zuo, K.; Li, L.; Liu, J.; Yuan, X.; Shen, Y.; Shao, M.; Pang, D.; Chu, Y.; et al. miR-23b promotes cutaneous wound healing through inhibition of the inflammatory responses by targeting ASK1. Acta Biochim. Biophys. Sin. 2018, 50, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-M.; Tao, J.; Chen, D.-D.; Cai, J.-J.; Irani, K.; Wang, Q.; Yuan, H.; Chen, A.F. MicroRNA miR-27b Rescues Bone Marrow–Derived Angiogenic Cell Function and Accelerates Wound Healing in Type 2 Diabetes Mellitus. Arter. Thromb. Vasc. Biol. 2014, 34, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Li, X.; Li, D.; Ren, X.; Li, Y.; Herter, E.K.; Qian, M.; Toma, M.-A.; Wintler, A.-M.; Sérézal, I.G.; et al. MicroRNA-34 Family Enhances Wound Inflammation by Targeting LGR4. J. Investig. Dermatol. 2020, 140, 465–476.e11. [Google Scholar] [CrossRef]
- Icli, B.; Wu, W.; Ozdemir, D.; Li, H.; Cheng, H.S.; Haemmig, S.; Liu, X.; Giatsidis, G.; Avci, S.N.; Lee, N.; et al. MicroRNA-615-5p Regulates Angiogenesis and Tissue Repair by Targeting AKT/eNOS (Protein Kinase B/Endothelial Nitric Oxide Synthase) Signaling in Endothelial Cells. Arter. Thromb. Vasc. Biol. 2019, 39, 1458–1474. [Google Scholar] [CrossRef]
- Jiang, F.-S.; Tian, S.-S.; Lu, J.; Ding, X.-H.; Qian, C.-D.; Ding, B.; Ding, Z.-S.; Jin, B. Cardamonin Regulates miR-21 Expression and Suppresses Angiogenesis Induced by Vascular Endothelial Growth Factor. BioMed Res. Int. 2015, 2015, 501581. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.; Serban, K.; Petrache, I. Effects of miR-126-enriched endothelial microparticles released during cigarette smoke exposure on endothelial cell migration and proliferation. In A28. EXOSOMES And MICRORNA; American Thoracic Society: New York, NY, USA, 2017; p. A1216. [Google Scholar]
- Suárez, Y.; Fernández-Hernando, C.; Pober, J.S.; Sessa, W. Dicer Dependent MicroRNAs Regulate Gene Expression and Functions in Human Endothelial Cells. Circ. Res. 2007, 100, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Li, X.; Wang, A.; Meisgen, F.; Pivarcsi, A.; Sonkoly, E.; Ståhle, M.; Landén, N.X. MicroRNA-31 Promotes Skin Wound Healing by Enhancing Keratinocyte Proliferation and Migration. J. Investig. Dermatol. 2015, 135, 1676–1685. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Wu, W.; Zheng, L.; Lin, X.; Tai, Y.; Wang, Y.; Wang, L. Roles of MicroRNA-21 in Skin Wound Healing: A Comprehensive Review. Front. Pharmacol. 2022, 13, 828627. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Wang, Y.; Li, L.; Wang, D.; Zhang, W.; Liu, L.; Jiang, H.; Yang, J.; Cheng, J. Overexpression of miR-29b reduces collagen biosynthesis by inhibiting heat shock protein 47 during skin wound healing. Transl. Res. 2016, 178, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Luo, X.; Wang, X.; Gao, Z. MicroRNA-185 regulates transforming growth factor-β1 and collagen-1 in hypertrophic scar fibroblasts. Mol. Med. Rep. 2017, 15, 1489–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghatak, S.; Chan, Y.C.; Khanna, S.; Banerjee, J.; Weist, J.; Roy, S.; Sen, C.K. Barrier Function of the Repaired Skin Is Disrupted Following Arrest of Dicer in Keratinocytes. Mol. Ther. 2015, 23, 1201–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, D.; Wang, A.; Chu, T.; Lohcharoenkal, W.; Zheng, X.; Grünler, J.; Narayanan, S.; Eliasson, S.; Herter, E.K.; et al. MicroRNA-132 with Therapeutic Potential in Chronic Wounds. J. Investig. Dermatol. 2017, 137, 2630–2638. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Feng, Y.; Sun, H.; Zhang, L.; Hao, L.; Shi, C.; Wang, J.; Li, R.; Ran, X.; Su, Y.; et al. miR-21 Regulates Skin Wound Healing by Targeting Multiple Aspects of the Healing Process. Am. J. Pathol. 2012, 181, 1911–1920. [Google Scholar] [CrossRef]
- Chun-Yuan, C.; Rao, S.-S.; Wang, Z.-X.; Cao, J.; Tan, Y.-J.; Luo, J.; Li, H.-M.; Zhang, W.-S.; Chen, C.-Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Wang, X.Y.; Zhou, L.Y.; Lao, G.J.; Liu, D.; Wang, C.; Hu, M.D.; Zeng, T.T.; Yan, L.; et al. MicroRNA-129 and -335 Promote Diabetic Wound Healing by Inhibiting Sp1-Mediated MMP-9 Expression. Diabetes 2018, 67, 1627–1638. [Google Scholar] [CrossRef] [Green Version]
- Ban, E.; Jeong, S.; Park, M.; Kwon, H.; Park, J.; Song, E.J.; Kim, A. Accelerated wound healing in diabetic mice by miRNA-497 and its anti-inflammatory activity. Biomed. Pharmacother. 2019, 121, 109613. [Google Scholar] [CrossRef]
- Zhong, H.; Qian, J.; Xiao, Z.; Chen, Y.; He, X.; Sun, C.; Zhao, Z. MicroRNA-133b Inhibition Restores EGFR Expression and Accelerates Diabetes-Impaired Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 9306760. [Google Scholar] [CrossRef]
- Hashempour, S.; Ghanbarzadeh, S.; I Maibach, H.; Ghorbani, M.; Hamishehkar, H. Skin toxicity of topically applied nanoparticles. Ther. Deliv. 2019, 10, 383–396. [Google Scholar] [CrossRef]
- Pan, Y.; Paschoalino, W.J.; Blum, A.S.; Mauzeroll, J. Recent Advances in Bio-Templated Metallic Nanomaterial Synthesis and Electrocatalytic Applications. ChemSusChem 2020, 14, 758–791. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2018, 119, 664–699. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chu, Z.; Wang, H.; Cai, L.; Tu, Z.; Liu, H.; Zhu, C.; Shi, H.; Pan, D.; Pan, J.; et al. Electrostatically Assembled Multilayered Films of Biopolymer Enhanced Nanocapsules for on-Demand Drug Release. ACS Appl. Bio Mater. 2019, 2, 3429–3438. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, J.; Zhang, Y.; Chen, G.; Mao, W.; Ye, Y.; Kahkoska, A.R.; Buse, J.B.; Langer, R.; Gu, Z. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat. Biomed. Eng. 2020, 4, 499–506. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.; Wen, D.; Wang, Z.; Li, H.; Zeng, Y.; Dotti, G.; Wirz, R.E.; Gu, Z. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 3687–3692. [Google Scholar] [CrossRef]
- Dukhinova, M.S.; Prilepskii, A.Y.; Shtil, A.A.; Vinogradov, V.V. Metal Oxide Nanoparticles in Therapeutic Regulation of Macrophage Functions. Nanomaterials 2019, 9, 1631. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Li, S.; Wang, H.; Cheng, W.; Li, Y.; Pan, L.; Shi, Y. Advanced electronic skin devices for healthcare applications. J. Mater. Chem. B 2018, 7, 173–197. [Google Scholar] [CrossRef]
- Leppiniemi, J.; Lahtinen, P.; Paajanen, A.; Mahlberg, R.; Metsä-Kortelainen, S.; Pinomaa, T.; Pajari, H.; Vikholm-Lundin, I.; Pursula, P.; Hytönen, V.P. 3D-Printable Bioactivated Nanocellulose–Alginate Hydrogels. ACS Appl. Mater. Interfaces 2017, 9, 21959–21970. [Google Scholar] [CrossRef] [Green Version]
- Okano, H.; Nakamura, M.; Yoshida, K.; Okada, Y.; Tsuji, O.; Nori, S.; Ikeda, E.; Yamanaka, S.; Miura, K. Steps Toward Safe Cell Therapy Using Induced Pluripotent Stem Cells. Circ. Res. 2013, 112, 523–533. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.K.; Parab, S.; Alexander, A.; Agrawal, M.; Achalla, V.P.K.; Pal, U.N.; Pandey, M.M.; Kesharwani, P. Cold atmospheric plasma therapy in wound healing. Process Biochem. 2021, 112, 112–123. [Google Scholar] [CrossRef]
- Oneto, P.; Etulain, J. PRP in wound healing applications. Platelets 2020, 32, 189–199. [Google Scholar] [CrossRef] [PubMed]






| Source of Stem Cells | Type of Wounds | Findings | Reference |
|---|---|---|---|
| Bone marrow-derived stem cells | Acute (full thickness wound) | Administration: intradermal and intravenous. Significant improvement in inflammation phase shortening, overexpression of proliferation markers (Ki67, CD71, and CD90), collagen deposition, and granulation tissue re-organization | [152] |
| Bone marrow-derived stem cells and their extracellular vesicles (EVs) | Acute (full thickness wound) | Administration: chitosan/collagen scaffold delivery system. Accelerated wound healing, enhanced collagen deposition | [153] |
| Bone marrow-derived stem cells | Chronic (diabetic wound) | Administration: subcutaneously. Improved collagen deposition and wound healing | [154] |
| Adipose-derived stem cells derived exosomes | Chronic (diabetic wound) | Upregulation and downregulation of specific micro RNAs (miRNAs), Inhibition of inflammation, modulation of PI3K/AKT signaling pathway | [155] |
| Adipose-derived stem cells | Chronic (full thickness burns wound) | Administration: 3D printed scaffold delivery system. Acceleration wound contraction, faster re-epithelialization and healing | [156] |
| Adipose-derived stem cells | Chronic (diabetic wound) | Administration: hydrogel delivery system. Enhanced neo-vascularization and accelerated wound closure | [157] |
| Hair follicles stem cells | Acute (full-thickness excisional wound) | Administration: intradermal injection. Shorter inflammation phase, function vascularization, enhanced re-epithelialization | [158] |
| Hair follicles stem cells | Chronic (venous leg ulcers) | Administration: direct application-hair skin graft. Significant reduction in ulcer area, improved healing | [159] |
| Hair follicles stem cells | Acute (full thickness skin wound) | Administration: direct application-hair skin graft. Overexpression of prostate cancer-upregulated long noncoding RNA 1 (PlncRNA-1), accelerated epidermal regeneration and wound healing | [160] |
| Induced pluripotent stem cells | Acute (full-thickness skin Wounds) | Administration: direct topical application. Expedited wound closure, enhanced collagen deposition | [161] |
| Induced pluripotent stem cell-derived exosomes | Chromic (diabetic ulcers) | Administration: direct. Enhanced migration and proliferation of fibroblasts, accelerated wound healing | [162] |
| Induced pluripotent stem cell-derived microvesicles | Chronic (burn wound) | Administration: Local transplantation. Accelerated wound closure, promotion of keratinocytes migration, increased re-epithelialization, | [163] |
| Biomaterial/Bioink/Cells | Bioprinting Method | Type of Wound | Findings | Reference |
|---|---|---|---|---|
| Fibrin and collagen hydrogel (Fibroblasts and keratinocytes) | In situ extrusion bioprinting | Acute (full thickness skin wound) | Rapid wound closure, reduced contraction, and accelerated re-epithelialization. | [215] |
| Fibrin hydrogel with gelatin, glycerol, and hyaluronic acid (Keratinocytes, melanocytes, fibroblasts, follicle dermal papilla cells, and microvascular endothelial cells, preadipocytes.) | Extrusion bioprinting | Acute (full thickness skin wound) | Accelerated wound closure, promotion of epidermal barrier formation, reduction in wounds contraction, remodeling of collagen | [223] |
| Gelatin/sodium alginate/gelatin methacrylate hydrogel (Dermal fibroblasts and epidermal keratinocytes) | Extrusion bioprinting | Acute (full thickness skin wound) | Reduced wound contraction and scarring, enhanced skin epithelialization, accelerated wound healing | [225] |
| Plasma-derived fibrinogen-containing factor XIII, fibronectin, thrombin, and macrophages (FPM bioink) (Primary fibroblasts human endothelial cells, and keratinocytes) | Extrusion bioprinting | Acute (full thickness skin wound) | Rapid wound closure and facilitation of re-epithelialization process | [226] |
| Fibrinogen/collagen hydrogel (Fibroblasts and keratinocytes) | In situ inkjet bioprinting | Acute (full thickness skin wound) | Improved wound closure and re-epithelialization process | [214] |
| Fibrin-collagen hydrogel (Amniotic fluid-derived stem (AFS) cells and bone marrow-derived mesenchymal stem cells (MSCs) | In situ extrusion bioprinting | Acute (full thickness skin wound) | Enhanced angiogenesis and wound closure rates | [227] |
| Skin-derived extracellular matrix (S-dECM) bio-ink (Fibroblasts, keratinocytes, endothelial progenitor cells and adipose-derived stem cells (ASCs) | Extrusion and inkjet bioprinting | Acute (full thickness skin wound) | Accelerated wound closure, enhanced re-epithelization, and neovascularization | [221] |
| Living photosynthetic microalgae scaffolds | In situ bioprinting | Chronic (diabetic wound) | Significantly reduced local hypoxia, accelerated chronic wound closure increased angiogenesis, and enhanced extracellular matrix (ECM) synthesis | [228] |
| Sodium alginate/gelatin/collagen hydrogel (Fibroblasts and keratinocytes) | Extrusion bioprinting | Acute (full thickness skin wound) | Enhanced re-epithelialization, reduced skin wound contraction, and accelerated wound healing | [229] |
| Strontium silicate (SS) microcylinders (Fibroblasts and keratinocytes) | Extrusion bioprinting | Acute and chronic wounds | Outstanding angiogenesis and wound healing | [230] |
| Type of Wounds | Number of Patients | Type of Plasma Treatment/Device/Injected Gas/Exposure Time | Findings | Reference |
|---|---|---|---|---|
| Chronic | n = 36 | MicroPlaSter cold plasma alpha device with argon (5 min daily treatment) | Significant bacterial load reduction (34%) | [295] |
| Chronic | n = 24 | MicroPlaSter cold plasma alpha device, MicroPlaSter cold plasma beta device with argon (2 min daily treatment) | Significant reduction in bacterial load (40%) | [296] |
| Chronic | n = 70 | MicroPlaSter cold plasma alpha device (3–7 min treatment) | Accelerated wound healing | [289] |
| Acute (wounds present at donor skin graft site) | n = 40 | Cold atmospheric argon plasma, Plasma jet with argon (2 min every day for 1 week) | Improved re-epithelialization at donor sites | [297] |
| Acute (trauma) | n = 2 | Plasma jet device with electrodes (20 min treatment) | Stopping of wound exudation, improved wound healing | [298] |
| Chronic (venous leg ulcers) | n = 14 | PlasmaDerm® VU-2010 device (45 s/cm2 for maximum 11 min thrice in a week for 8 weeks) | Strong antibacterial effects, significant reduction in chronic ulcer size | [299] |
| Chronic (venous leg ulcers) | n = 16 | Antiseptic effects of cold atmospheric pressure plasma (APP) or octenidine (OCT) with argon | Significant microbial reduction (64%) without cytotoxicity | [300] |
| Chronic | n = 34 | Tissue-tolerable plasma (TTP) and modern conventional liquid antiseptics | Provided most efficient strategy using antiseptic treatment, highest antimicrobial efficacy, | [301] |
| Chronic (pressure ulcers) | n = 50 | Low-temperature atmospheric-pressure plasma (LTAPP) jet with argon (1 minute/cm2 once in a week for 8 weeks) | Reduction in bacterial load, significantly better PUSH (Pressure Ulcer Scale for Healing) score | [292] |
| Chronic (diabetic foot) | n = 65 | Argon Plasma Jet (8 times treatment within 14 days) | Reduction in wound surface area, change in microbial load | [302] |
| Chronic (diabetic wounds) | n = 14 | Cold atmospheric plasma | Promotion of vascularization, granulation tissue formation, and re-epithelialization | [286] |
| Wound Healing Phase | microRNAs (miRs) | Function | Effect on Wound Healing | Reference |
|---|---|---|---|---|
| Inflammation | miR-142-3p/5p | Promotion of neutrophils migration | Promote wound healing | [311] |
| Inflammation | miR-203 | Alleviation of skin inflammation | Promote wound healing | [312] |
| Inflammation | miR-23b | Escalation of of anti-inflammatory cytokines and reduction of pro-inflammatory cytokines | Promote wound healing | [313] |
| Inflammation | miR-27b | Reduce ROS production | Promote wound healing | [314] |
| Inflammation | miR-34 | Enhancement of NF-κB signaling pathway activity | Impede wound healing | [315] |
| Angiogenesis | miR-615-5p | Inhibition of angiogenesis by targeting protein kinase B/endothelial nitric oxide synthase signaling pathway | Impede wound healing | [316] |
| Angiogenesis | miR-21 | Suppression of angiogenesis by downregulating the expression of tensin homolog (PTEN) and SMAD7 genes | Impede wound healing | [317] |
| Angiogenesis | miR-126 | Enhances migration and repair of endothelial cells | Promote wound healing | [318] |
| Angiogenesis | miR-221 and miR-222 | The expression of endothelial NO | Impede wound healing | [319] |
| Re-epithelialization | miR-31 | Enhances keratinocytes proliferation and migration | Promote wound healing | [320] |
| Re-epithelialization | miR-21 | Promotes fibroblasts differentiation, collagen synthesis, and re-epithelialization | Promote wound healing | [321] |
| Granulation tissue formation | miR-29b | Inhibits expression of heat shock protein 47 (HSP47) and collagen synthesis | Impede wound healing | [322] |
| Granulation tissue formation | miR-185 | Inhibits fibroblasts growth and function | Impede wound healing | [323] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. https://doi.org/10.3390/cells11152439
Kolimi P, Narala S, Nyavanandi D, Youssef AAA, Dudhipala N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells. 2022; 11(15):2439. https://doi.org/10.3390/cells11152439
Chicago/Turabian StyleKolimi, Praveen, Sagar Narala, Dinesh Nyavanandi, Ahmed Adel Ali Youssef, and Narendar Dudhipala. 2022. "Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements" Cells 11, no. 15: 2439. https://doi.org/10.3390/cells11152439
APA StyleKolimi, P., Narala, S., Nyavanandi, D., Youssef, A. A. A., & Dudhipala, N. (2022). Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells, 11(15), 2439. https://doi.org/10.3390/cells11152439