Allergen Immunotherapy: Current and Future Trends
Abstract
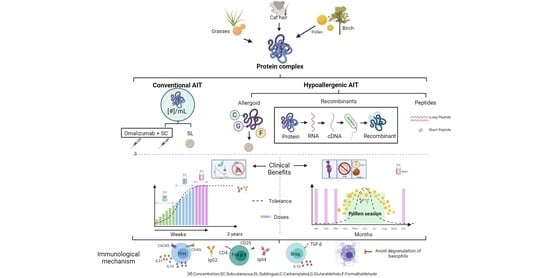
:1. Introduction
2. Mechanisms of Action of AIT
3. Efficacy of Allergen Immunotherapy
4. Allergoids
5. Adjuvants
5.1. Aluminum
5.2. Microcrystalline Tyrosine (MCT)
5.3. Calcium Phosphate (CaP)
5.4. Toll-like Receptors (TLR)
5.5. Liposomes
5.6. Virus-like Particles (VLPs)
6. Peptides and Recombinants
7. Clinical-Immune Efficacy of Recombinant Allergens
7.1. Cat
7.2. Birch
7.3. Grasses
8. Passive Immunization with IgG Antibodies
9. Benefits and Limitations of Novel Immunotherapies
10. Recombinants for Diagnosis
11. Allergy Proteomics
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ring, J.; Gutermuth, J. 100 years of hyposensitization: History of allergen-specific immunotherapy (ASIT). Allergy 2011, 66, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Frankland, A.W.; Augustin, R. Prophylaxis of summer hay-fever and asthma. Lancet 1954, 263, 1055–1057. [Google Scholar] [CrossRef]
- Bousquet, J.; Schünemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J. Allergy Clin. Immunol. 2020, 145, 70–80.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Initiative of Asthma. Global Strategy for Asthma Management and Prevention. GINA. 2021. Available online: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf (accessed on 15 September 2021).
- Pfaar, O.; Bachert, C.; Bufe, A.; Buhl, R.; Ebner, C.; Eng, P.; Friedrichs, F.; Fuchs, T.; Hamelmann, E.; Hartwig-Bade, D.; et al. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases. Allergo J. Int. 2014, 23, 282–319. [Google Scholar] [CrossRef]
- Durham, S.R.; Walker, S.M.; Varga, E.-M.; Jacobson, M.R.; O’Brien, F.; Noble, W.; Till, S.J.; Hamid, Q.A.; Nouri-Aria, K.T. Long-Term Clinical Efficacy of Grass-Pollen Immunotherapy. N. Engl. J. Med. 1999, 341, 468–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penagos, M.; Durham, S.R. Duration of allergen immunotherapy for inhalant allergy. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 594–605. [Google Scholar] [CrossRef]
- Pavon-Romero, G.F.; Larenas-Linnemann, D.E.; Xochipa Ruiz, K.K.E.; Ramirez-Jimenez, F.; Teran, L.M.; Ramirez-Jimenez, F.; Teran, L. Subcutaneous Allergen-Specific Immunotherapy Is Safe in Pediatric Patients with Allergic Rhinitis. Int. Arch. Allergy Immunol. 2021, 182, 553–561. [Google Scholar] [CrossRef]
- Larenas-Linnemann, D.; Luna-Pech, J.; Rodríguez-Pérez, N.; Rodríguez-González, M.; Arias-Cruz, A.; Blandón-Vijil, M.; Costa-Domínguez, M.; Del Río-Navarro, B.; Estrada-Cardona, A.; Navarrete-Rodríguez, E.; et al. GUIMIT 2019, Mexican Guideline on Immunotherapy. Guideline on the diagnosis of IgE-mediated allergic disease and immunotherapy following the ADAPTE approach. Rev. Alerg. Mex. 2019, 66 (Suppl. S1), 1–105. [Google Scholar] [CrossRef]
- Gardner, L.; Thien, F.; Douglass, J.; Rolland, J.; O’Hehir, R. Induction of T “regulatory” cells by standardized house dust mite immunotherapy: An increase in CD4+CD25+ interleukin-10+ T cells expressing peripheral tissue trafficking markers. Clin. Exp. Allergy 2004, 34, 1209–1219. [Google Scholar] [CrossRef]
- Varona, R.; Ramos, T.; Escribese, M.; Jimeno, L.; Galán, A.; Würtzen, P.; Vega, F.; Marín, A.; Martín, S.; Carrera, A.; et al. Persistent regulatory T-cell response 2 years after 3 years of grass tablet SLIT: Links to reduced eosinophil counts, sIgE levels, and clinical benefit. Allergy 2019, 74, 349–360. [Google Scholar] [CrossRef]
- O’Hehir, R.; Gardner, L.; de Leon, M.; Hales, B.; Biondo, M.; Douglass, J.; Rolland, J.; Sandrini, A. House dust mite sublingual immu-notherapy: The role for transforming growth factor-beta and functional regulatory T cells. Am. J. Respir. Crit. Care Med. 2009, 180, 936–947. [Google Scholar] [CrossRef]
- Gardner, L.; Rolland, J.M.; O’Hehir, R.E. High Dose Allergen Stimulation of T Cells from House Dust Mite-Allergic Subjects Induces Expansion of IFN-?+ T Cells, Apoptosis of CD4+IL-4+ T Cells and T Cell Anergy. Int. Arch. Allergy Immunol. 2004, 133, 1–13. [Google Scholar] [CrossRef]
- Francis, J.N.; Till, S.J.; Durham, S.R. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J. Allergy Clin. Immunol. 2003, 111, 1255–1261. [Google Scholar] [CrossRef]
- Royer, B.; Varadaradjalou, S.; Saas, P.; Guillosson, J.J.; Kantelip, J.P.; Arock, M. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin. Exp. Allergy 2001, 31, 694–704. [Google Scholar] [CrossRef]
- Rak, S.; Löwhagen, O.; Venge, P. The effect of immunotherapy on bronchial hyperresponsiveness and eosinophil cationic protein in pollen-allergic patients. J. Allergy Clin. Immunol. 1988, 82, 470–480. [Google Scholar] [CrossRef]
- Wachholz, P.A.; Soni, N.K.; Till, S.J.; Durham, S.R. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J. Allergy Clin. Immunol. 2003, 112, 915–922. [Google Scholar] [CrossRef]
- Scadding, G.W.; Shamji, M.H.; Jacobson, M.R.; Lee, D.I.; Wilson, D.; Lima, M.T.; Pitkin, L.; Pilette, C.; Nouri-Aria, K.; Durham, S.R. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin. Exp. Allergy 2010, 40, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Linterman, M.A.; Pierson, W.; Lee, S.K.; Kallies, A.; Kawamoto, S.; Rayner, T.F.; Srivastava, M.; Divekar, D.P.; Beaton, L.; Hogan, J.J.; et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 2011, 17, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Shulman, Z.; Gitlin, A.D.; Weinstein, J.S.; Lainez, B.; Esplugues, E.; Flavell, R.A.; Craft, J.E.; Nussenzweig, M.C. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science 2014, 345, 1058–1062. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M.; Baba, A.; Yokota, T.; Nishikawa, H.; Ohkawa, Y.; Kayama, H.; Kallies, A.; Nutt, S.L.; Sakaguchi, S.; Takeda, K.; et al. Interleukin-10-Producing Plasmablasts Exert Regulatory Function in Autoimmune Inflammation. Immunity 2014, 41, 1040–1051. [Google Scholar] [CrossRef] [Green Version]
- Xian, M.; Feng, M.; Dong, Y.; Wei, N.; Su, Q.; Li, J. Changes in CD4+CD25+FoxP3+ Regulatory T Cells and Serum Cytokines in Sublingual and Subcutaneous Immunotherapy in Allergic Rhinitis with or without Asthma. Int. Arch. Allergy Immunol. 2019, 181, 71–80. [Google Scholar] [CrossRef]
- Nouri-Aria, K.T.; Wachholz, P.A.; Francis, J.N.; Jacobson, M.R.; Walker, S.M.; Wilcock, L.K.; Staple, S.Q.; Aalberse, R.C.; Till, S.J.; Durham, S.R. Grass Pollen Immunotherapy Induces Mucosal and Peripheral IL-10 Responses and Blocking IgG Activity. J. Immunol. 2004, 172, 3252–3259. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Fueyo, A.; Ramos, T.; Galán, A.; Jimeno, L.; Wurtzen, P.A.; Marin, A.; de Frutos, C.; Blanco, C.; Carrera, A.; Barber, D.; et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. J. Allergy Clin. Immunol. 2014, 133, 130–138.e2. [Google Scholar] [CrossRef]
- Bohle, B.; Kinaciyan, T.; Gerstmayr, M.; Radakovics, A.; Jahn-Schmid, B.; Ebner, C. Sublingual immunotherapy induces IL-10–producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J. Allergy Clin. Immunol. 2007, 120, 707–713. [Google Scholar] [CrossRef]
- Allam, J.-P.; Peng, W.-M.; Appel, T.; Wenghoefer, M.; Niederhagen, B.; Bieber, T.; Bergé, S.; Novak, N. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J. Allergy Clin. Immunol. 2008, 121, 368–374.e1. [Google Scholar] [CrossRef]
- Ihara, F.; Sakurai, T.; Yonekura, S.; Iinuma, T.; Yagi, R.; Ito, T.; Matsuura, A.; Morimoto, Y.; Arai, T.; Suzuki, S.; et al. Identification of specifically reduced Th2 cell subsets in allergic rhinitis patients after sublingual immunotherapy. Allergy 2018, 73, 1823–1832. [Google Scholar] [CrossRef]
- Rotiroti, G.; Shamji, M.; Durham, S.R.; Till, S.J. Repeated low-dose intradermal allergen injection suppresses allergen-induced cutaneous late responses. J. Allergy Clin. Immunol. 2012, 130, 918–924.e1. [Google Scholar] [CrossRef]
- Heeringa, J.J.; McKenzie, C.I.; Varese, N.; Hew, M.; Bakx, A.T.C.M.; Aui, P.M.; Rolland, J.M.; O’Hehir, R.E.; Van Zelm, M.C. Induction of IgG 2 and IgG 4 B-cell memory following sublingual immunotherapy for ryegrass pollen allergy. Allergy 2020, 75, 1121–1132. [Google Scholar] [CrossRef] [Green Version]
- Couroux, P.; Ipsen, H.; Stage, B.S.; Damkjaer, J.T.; Steffensen, M.A.; Salapatek, A.M.; Lund, K.; Würtzen, P.A. A birch sublingual allergy immunotherapy tablet reduces rhinoconjunctivitis symptoms when exposed to birch and oak and induces IgG4 to allergens from all trees in the birch homologous group. Allergy 2019, 74, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Senti, G.; Graf, N.; Haug, S.; Rüedi, N.; von Moos, S.; Sonderegger, T.; Johansen, P.; Kündig, T.M. Epicutaneous allergen administration as a novel method of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2009, 124, 997–1002. [Google Scholar] [CrossRef]
- Senti, G.; Von Moos, S.; Tay, F.; Graf, N.; Johansen, P.; Kündig, T.M. Determinants of efficacy and safety in epicutaneous allergen immunotherapy: Summary of three clinical trials. Allergy 2015, 70, 707–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strid, J.; Hourihane, J.; Kimber, I.; Callard, R.; Strobel, S. Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. Eur. J. Immunol. 2004, 34, 2100–2109. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, J.M.; Johansen, P.; Erdmann, I.; Senti, G.; Crameri, R.; Kündig, T.M. Intralymphatic Injections as a New Administration Route for Allergen-Specific Immunotherapy. Int. Arch. Allergy Immunol. 2009, 150, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senti, G.; Vavricka, B.M.P.; Erdmann, I.; Diaz, M.I.; Markus, R.; McCormack, S.J.; Simard, J.J.; Wüthrich, B.; Crameri, R.; Graf, N.; et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: A randomized controlled trial. Proc. Natl. Acad. Sci. USA 2008, 105, 17908–17912. [Google Scholar] [CrossRef] [Green Version]
- Virchow, J.C.; Backer, V.; Kuna, P.; Prieto, L.; Nolte, H.; Villesen, H.H.; Ljørring, C.; Riis, B.; De Blay, F. Efficacy of a House Dust Mite Sublingual Allergen Immunotherapy Tablet in Adults With Allergic Asthma. JAMA 2016, 315, 1715–1725. [Google Scholar] [CrossRef]
- Fortescue, R.; Kew, K.M.; Leung, M.S.T. Sublingual immunotherapy for asthma. Cochrane Database Syst. Rev. 2020, 9, CD011293–CD011293. [Google Scholar] [CrossRef]
- Calderón, M.A.; Alves, B.; Jacobson, M.; Hurwitz, B.; Sheikh, A.; Durham, S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst. Rev. 2007, 2007, CD001936. [Google Scholar] [CrossRef]
- Dhami, S.; Nurmatov, U.; Arasi, S.; Khan, T.; Asaria, M.; Zaman, H.; Agarwal, A.; Netuveli, G.; Roberts, G.; Pfaar, O.; et al. Allergen immunotherapy for allergic rhinoconjunctivitis: A systematic review and meta-analysis. Allergy 2017, 72, 1597–1631. [Google Scholar] [CrossRef] [Green Version]
- Coop, C.A. Immunotherapy for Mold Allergy. Clin. Rev. Allergy Immunol. 2013, 47, 289–298. [Google Scholar] [CrossRef]
- Bozek, A.; Pyrkosz, K. Immunotherapy of mold allergy: A review. Hum. Vaccines Immunother. 2017, 13, 2397–2401. [Google Scholar] [CrossRef]
- Radulovic, S.; Calderón, M.A.; Wilson, D.; Durham, S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst. Rev. 2010, 2010, 002893. [Google Scholar] [CrossRef]
- Sieber, J.; Shah-Hosseini, K.; Mösges, R. Specific immunotherapy for allergic rhinitis to grass and tree pollens in daily medical practice—Symptom load with sublingual immunotherapy compared to subcutaneous immunotherapy. Ann. Med. 2011, 43, 418–424. [Google Scholar] [CrossRef]
- Nelson, H.; Cartier, S.; Allen-Ramey, F.; Lawton, S.; Calderon, M.A. Network Meta-analysis Shows Commercialized Subcutaneous and Sublingual Grass Products Have Comparable Efficacy. J. Allergy Clin. Immunol. Pract. 2015, 3, 256–266.e3. [Google Scholar] [CrossRef]
- Dhami, S.; Agarwal, A. Does evidence support the use of cat allergen immunotherapy? Curr. Opin. Allergy Clin. Immunol. 2018, 18, 350–355. [Google Scholar] [CrossRef]
- Calderon, M.A.; Casale, T.B.; Nelson, H.S.; Demoly, P. An evidence-based analysis of house dust mite allergen immunotherapy: A call for more rigorous clinical studies. J. Allergy Clin. Immunol. 2013, 132, 1322–1336. [Google Scholar] [CrossRef]
- Marogna, M.; Spadolini, I.; Massolo, A.; Canonica, G.W.; Passalacqua, G. Long-lasting effects of sublingual immunotherapy according to its duration: A 15-year prospective study. J. Allergy Clin. Immunol. 2010, 126, 969–975. [Google Scholar] [CrossRef]
- Marogna, M.; Bruno, M.; Massolo, A.; Falagiani, P. Long-Lasting Effects of Sublingual Immunotherapy for House Dust Mites in Allergic Rhinitis with Bronchial Hyperreactivity: A Long-Term (13-Year) Retrospective Study in Real Life. Int. Arch. Allergy Immunol. 2006, 142, 70–78. [Google Scholar] [CrossRef]
- Pajno, G.B.; Barberio, G.; De Luca, F.; Morabito, L.; Parmiani, S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin. Exp. Allergy 2001, 31, 1392–1397. [Google Scholar] [CrossRef]
- Inal, A.; Altintas, D.; Yilmaz, M.; Karakoc, G.; Kendirli, S.; Sertdemir, Y. Prevention of new sensitizations by specific immunotherapy in children with rhinitis and/or asthma monosensitized to house dust mite. J. Investig. Allergol. Clin. Immunol. 2007, 17, 85–91. [Google Scholar]
- Tabar, A.I.; Prieto, L.; Alba, P.; Nieto, A.; Rodríguez, M.; Torrecillas, M.; Huertas, B.; Gómez, E.; Fernández, F.J.; Blanca, M.; et al. Double-blind, randomized, placebo-controlled trial of allergen-specific immunotherapy with the major allergen Alt a 1. J. Allergy Clin. Immunol. 2019, 144, 216–223.e3. [Google Scholar] [CrossRef] [Green Version]
- Soyyigit, S.; Guloglu, D.; Ikinciogullari, A.; Secil, D.; Oztuna, D.; Mungan, D.; Misirligil, Z.; Sin, B.A. Immunologic alterations and efficacy of subcutaneous immunotherapy with Dermatophagoides pteronyssinus in monosensitized and polysensitized patients. Ann. Allergy Asthma Immunol. 2016, 116, 244–251.e2. [Google Scholar] [CrossRef]
- Kucuksezer, U.C.; Ozdemir, C.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol. Int. 2020, 69, 549–560. [Google Scholar] [CrossRef]
- Özdemir, S.K.; Sin, B.A.; Güloğlu, D.; Ikincioğulları, A.; Gençtürk, Z.; Mısırlıgil, Z. Short-Term Preseasonal Immunotherapy: Is Early Clinical Efficacy Related to the Basophil Response? Int. Arch. Allergy Immunol. 2014, 164, 237–245. [Google Scholar] [CrossRef]
- Epstein, T.G.; Liss, G.M.; Murphy-Berendts, K.; Bernstein, D.I. Risk factors for fatal and nonfatal reactions to subcutaneous immunotherapy. Ann. Allergy Asthma Immunol. 2016, 116, 354–359.e2. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Wanner, M.; Borish, L.; Liss, G.M.; The Immunotherapy Committee of the American Academy of Allergy, Asthma and Immunology. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990–2001. J. Allergy Clin. Immunol. 2004, 113, 1129–1136. [Google Scholar] [CrossRef]
- Casale, T.B.; Busse, W.W.; Kline, J.; Ballas, Z.; Moss, M.H.; Townley, R.G.; Mokhtarani, M.; Seyfert-Margolis, V.; Asare, A.; Bateman, K. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2006, 117, 134–140. [Google Scholar] [CrossRef]
- Kuehr, J.; Brauburger, J.; Zielen, S.; Schauer, U.; Kamin, W.; Von Berg, A.; Leupold, W.; Bergmann, K.-C.; Rolinck-Werninghaus, C.; Gräve, M.; et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2002, 109, 274–280. [Google Scholar] [CrossRef]
- Kopp, M.V.; Hamelmann, E.; Zielen, S.; Kamin, W.; Bergmann, K.-C.; Sieder, C.; Stenglein, S.; Seyfried, S.; Wahn, U.; for The DUAL Study Group. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin. Exp. Allergy 2009, 39, 271–279. [Google Scholar] [CrossRef]
- Klimek, L.; Thorn, C.; Pfaar, O. Depigmentierte Allergoide für die allergenspezifische Immuntherapie. HNO 2010, 58, 51–56. [Google Scholar] [CrossRef]
- Ibarrola, I.; Sanz, M.L.; Gamboa, P.M.; Mir, A.; Benahmed, D.; Ferrer, A.; Arilla, M.C.; Martinez, A.; Asturias, J. Biological characterization of glutaraldehyde-modified Parietaria judaica pollen extracts. Clin. Exp. Allergy 2004, 34, 303–309. [Google Scholar] [CrossRef]
- Würtzen, P.A.; Lund, L.; Lund, G.; Holm, J.; Millner, A.; Henmar, H. Chemical Modification of Birch Allergen Extract Leads to a Reduction in Allergenicity as well as Immunogenicity. Int. Arch. Allergy Immunol. 2007, 144, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Gokmen, N.M.; Ersoy, R.; Gulbahar, O.; Ardeniz, O.; Sin, A.; Unsel, M.; Kokuludag, A. Desensitization Effect of Preseasonal Seven-Injection Allergoid Immunotherapy with Olive Pollen on Basophil Activation: The Efficacy of Olive Pollen-Specific Preseasonal Allergoid Immunotherapy on Basophils. Int. Arch. Allergy Immunol. 2012, 159, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Mahler, V.; Zielen, S.; Rosewich, M. Year-round treatment initiation for a 6-grasses pollen allergoid in specific immunotherapy of allergic rhinoconjunctivitis and asthma. Immunotherapy 2019, 11, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, C.; Kettner, J.; Doemer, C.; Cromwell, O.; Narkus, A.; the Study Group. Efficacy and safety of preseasonal-specific immunotherapy with an aluminium-adsorbed six-grass pollen allergoid. Allergy 2005, 60, 801–807. [Google Scholar] [CrossRef]
- Pfaar, O.; Hohlfeld, J.M.; Al-Kadah, B.; Hauswald, B.; Homey, B.; Hunzelmann, N.; Schliemann, S.; Velling, P.; Worm, M.; Klimek, L. Dose-response relationship of a new Timothy grass pollen allergoid in comparison with a 6-grass pollen allergoid. Clin. Exp. Allergy 2017, 47, 1445–1455. [Google Scholar] [CrossRef] [Green Version]
- Pfaar, O.; Nell, M.J.; Boot, J.D.; Versteeg, S.A.; van Ree, R.; Roger, A.; Riechelmann, H.; Sperl, A.; Elberink, J.N.G.O.; Diamant, Z.; et al. A randomized, 5-arm dose finding study with a mite allergoid SCIT in allergic rhinoconjunctivitis patients. Allergy 2016, 71, 967–976. [Google Scholar] [CrossRef]
- Jutel, M.; Rudert, M.; Kreimendahl, F.; Kuna, P. Efficacy and tolerability of a house dust mite allergoid in allergic bronchial asthma: A randomized dose-ranging trial. Immunotherapy 2018, 10, 1149–1161. [Google Scholar] [CrossRef]
- Zielen, S.; Plückhahn, K.; Akboga, Y.; Rieker-Schwienbacher, J.; Thieme, U.; Rosewich, M. Fast up-dosing with a birch allergoid is safe and well tolerated in allergic rhinitis patients with or without asthma. Immunotherapy 2019, 11, 177–187. [Google Scholar] [CrossRef]
- Buczyłko, K.; Boot, D.; Van Ree, R.; Van Der Werf, J.F. Accelerated Up-Dosing of Subcutaneous Immunotherapy with a Registered Allergoid Birch Pollen Preparation. Int. Arch. Allergy Immunol. 2017, 172, 183–186. [Google Scholar] [CrossRef]
- Durham, S.R. Sustained effects of grass pollen AIT. Allergy 2011, 66, 50–52. [Google Scholar] [CrossRef]
- Mösges, R.; Ritter, B.; Kayoko, G.; Allekotte, S. Carbamylated monomeric allergoids as a therapeutic option for sublingual immunotherapy of dust mite- and grass pollen-induced allergic rhinoconjunctivitis: A systematic review of published trials with a meta-analysis of treatment using Lais® tablets. Acta Dermatovenerol. Alpina Pannonica Adriat. 2010, 19, 1–8. [Google Scholar]
- Durham, S.R.; Emminger, W.; Kapp, A.; de Monchy, J.G.; Rak, S.; Scadding, G.K.; Wurtzen, P.A.; Andersen, J.S.; Tholstrup, B.; Riis, B.; et al. SQ-standardized sublingual grass immunotherapy: Confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J. Allergy Clin. Immunol. 2012, 129, 717–725.e5. [Google Scholar] [CrossRef] [Green Version]
- Di Gioacchino, M.; Cavallucci, E.; Ballone, E.; Cervone, M.; Di Rocco, P.; Piunti, E.; Filardo, G.; Turi, M.; Mangifesta, R.; Quecchia, C.; et al. Dose-Dependent Clinical and Immunological Efficacy of Sublingual Immunotherapy with Mite Monomeric Allergoid. Int. J. Immunopathol. Pharmacol. 2012, 25, 671–679. [Google Scholar] [CrossRef]
- Hüser, C.; Dieterich, P.; Singh, J.; Shah-Hosseini, K.; Allekotte, S.; Lehmacher, W.; Compalati, E.; Mösges, R. A 12-week DBPC dose-finding study with sublingual monomeric allergoid tablets in house dust mite-allergic patients. Allergy 2016, 72, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Kopp, M.V.; Bovermann, X.; Klimek, L. Accelerated Dose Escalation with Three Injections of an Aluminum Hydroxide-Adsorbed Allergoid Preparation of Six Grasses Is Safe for Patients with Moderate to Severe Allergic Rhinitis. Int. Arch. Allergy Immunol. 2019, 181, 94–102. [Google Scholar] [CrossRef]
- Chaker, A.M.; Al-Kadah, B.; Luther, U.; Neumann, U.; Wagenmann, M. An accelerated dose escalation with a grass pollen allergoid is safe and well-tolerated: A randomized open label phase II trial. Clin. Transl. Allergy 2015, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Vinay, T.-N.; Park, C.-S.; Kim, H.-Y.; Jung, S.-J. Toxicity and dose determination of quillaja saponin, aluminum hydroxide and squalene in olive flounder (Paralichthys olivaceus). Vet. Immunol. Immunopathol. 2014, 158, 73–85. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- Mannhalter, J.; Neychev, H.; Zlabinger, G.; Ahmad, R.; Eibl, M. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: Effect on antigen uptake and antigen presentation. Clin. Exp. Immunol. 1985, 61, 143. [Google Scholar]
- Güven, E.; Duus, K.; Laursen, I.; Højrup, P.; Houen, G. Aluminum Hydroxide Adjuvant Differentially Activates the Three Complement Pathways with Major Involvement of the Alternative Pathway. PLoS ONE 2013, 8, e74445. [Google Scholar] [CrossRef]
- Eisenbarth, S.; Colegio, O.; O’Connor, W.; Sutterwala, F.S.; Flavell, R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nat. Cell Biol. 2008, 453, 1122–1126. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Kool, M.; Willart, M.A.; Hammad, H. Mechanism of action of clinically approved adjuvants. Curr. Opin. Immunol. 2009, 21, 23–29. [Google Scholar] [CrossRef]
- Zaki, S.R.; Austin, G.E.; Chan, W.C.; Conaty, A.L.; Trusler, S.; Trappier, S.; Lindsey, R.B.; Swan, D.C. Chromosomal localization of the human myeloperoxidase gene by in situ hybridization using oligonucleotide probes. Genes Chromosom. Cancer 1990, 2, 266–270. [Google Scholar] [CrossRef]
- Gadermaier, E.; Flicker, S.; Aberer, W.; Egger, C.; Reider, N.; Focke, M.; Vrtala, S.; Kundi, M.; Valenta, R. Analysis of the Antibody Responses Induced by Subcutaneous Injection Immunotherapy with Birch and Fagales Pollen Extracts Adsorbed onto Aluminum Hydroxide. Int. Arch. Allergy Immunol. 2010, 151, 17–27. [Google Scholar] [CrossRef]
- Spertini, F.; DellaCorte, G.; Kettner, A.; de Blay, F.; Jacobsen, L.; Jutel, M.; Worm, M.; Charlon, V.; Reymond, C. Efficacy of 2 months of allergen-specific immunotherapy with Bet v 1–derived contiguous overlapping peptides in patients with allergic rhinoconjunctivitis: Results of a phase IIb study. J. Allergy Clin. Immunol. 2016, 138, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Fulgencio, M.; Caballero, R.; Lara, B.; Mena, M.; Tejera, M.; Sastre, A.; Subiza, J.-L.; Fernández-Caldas, E.; Casanovas, M. Safety of immunotherapy with glutaraldehyde modified allergen extracts in children and adults. Allergol. Immunopathol. 2017, 45, 198–207. [Google Scholar] [CrossRef]
- Garcí-Patos, V.; Pujol, R.M.; Alomar, A.; Cisteró, A.; Curell, R.; Fernández-Figueras, M.T.; de Moragas, J.M. Persistent Subcutaneous Nodules in Patients Hyposensitized With Aluminum-Containing Allergen Extracts. Arch. Dermatol. 1995, 131, 1421–1424. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S. Aluminum in vaccines: Does it create a safety problem? Vaccine 2018, 36, 5825–5831. [Google Scholar] [CrossRef]
- Baldrick, P.; Richardson, D.; Wheeler, A.W. Review ofL-tyrosine confirming its safe human use as an adjuvant. J. Appl. Toxicol. 2002, 22, 333–344. [Google Scholar] [CrossRef]
- Wheeler, A.; Moran, D.; Robins, B.; Driscoll, A. L-Tyrosine as an Immunological Adjuvant. Int. Arch. Allergy Immunol. 1982, 69, 113–119. [Google Scholar] [CrossRef]
- McDougall, S.A.; Heath, M.D.; Kramer, M.F.; Skinner, M.A. Analysis of aluminium in rat following administration of allergen immunotherapy using either aluminium or microcrystalline-tyrosine-based adjuvants. Bioanalysis 2016, 8, 547–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, A.; Heath, M.; Hewings, S.; Skinner, M. The adsorption of allergoids and 3-O-desacyl-4′-monophosphoryl lipid A (MPL®) to microcrystalline tyrosine (MCT) in formulations for use in allergy immunotherapy. J. Inorg. Biochem. 2015, 152, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, A.; Malet, A.; Moreno, V.; Parra, A.; Gutiérrez, D.; Lleonart, R.; Moreno, F.; Valero, A.; Navarro, B.; Hinojosa, B.; et al. Real-life effect of a microcrystalline tyrosine adjuvanted mite immunotherapy in patients with allergic rhinitis. Immunotherapy 2020, 12, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Roger, A.; Depreux, N.; Jurgens, Y.; Serra, A.T.; Heath, M.D.; Garcia, G.; Skinner, M.A. A novel microcrystalline tyrosine-adsorbed, mite-allergoid subcutaneous immunotherapy: 1-year follow-up report. Immunotherapy 2016, 8, 1169–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, A.; Depreux, N.; Jurgens, Y.; Heath, M.D.; Garcia, G.; Skinner, M.A. A novel and well tolerated mite allergoid subcutaneous immunotherapy: Evidence of clinical and immunologic efficacy. Immun. Inflamm. Dis. 2014, 2, 92–98. [Google Scholar] [CrossRef]
- Becker, S.; Zieglmayer, P.; Canto, G.; Fassio, F.; Yong, P.; Acikel, C.; Raskopf, E.; Steveling-Klein, E.H.; Allekotte, S.; Mösges, R. A meta-analysis on allergen-specific immunotherapy using MCT ® (MicroCrystalline Tyrosine)-adsorbed allergoids in pollen allergic patients suffering from allergic rhinoconjunctivitis. Clin. Transl. Allergy 2021, 11, e12037. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information Calcium Phosphate | Ca3(PO4)2—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Calcium-phosphate (accessed on 12 September 2021).
- Jones, S.; Asokanathan, C.; Kmiec, D.; Irvine, J.; Fleck, R.; Xing, D.; Moore, B.; Parton, R.; Coote, J. Protein coated microcrystals formulated with model antigens and modified with calcium phosphate exhibit enhanced phagocytosis and immunogenicity. Vaccine 2013, 32, 4234–4242. [Google Scholar] [CrossRef] [Green Version]
- Leynadier, F.; Banoun, L.; Dollois, B.; Terrier, P.; Epstein, M.; Guinnepain, M.-T.; Firon, D.; Traube, C.; Fadel, R.; Andre, C. Immunotherapy with a calcium phosphate-adsorbed five-grass-pollen extract in seasonal rhinoconjunctivitis: A double-blind, placebo-controlled study. Clin. Exp. Allergy 2001, 31, 988–996. [Google Scholar] [CrossRef]
- Masson, J.-D.; Thibaudon, M.; Bélec, L.; Crépeaux, G. Calcium phosphate: A substitute for aluminum adjuvants? Expert Rev. Vaccines 2016, 16, 289–299. [Google Scholar] [CrossRef]
- Kartal, O.; Güleç, M.; Caliskaner, Z.; Musabak, U.H.; Şener, O. Safety of subcutaneous immunotherapy with inhalant allergen extracts: A single-center 30-year experience from Turkey. Immunopharmacol. Immunotoxicol. 2015, 37, 280–286. [Google Scholar] [CrossRef]
- Boghdadi, G.; Hammad, N.; Amer, A.; Sammour, S.; Sorour, S. R848, a Toll-Like Receptors 7 and 8 Agonist, a Potential Therapy for Allergic Rhinitis Patients. Inflamm. Allergy—Drug Targets 2014, 13, 144–149. [Google Scholar] [CrossRef]
- Fuchs, B.; Knothe, S.; Rochlitzer, S.; Nassimi, M.; Greweling, M.; Lauenstein, H.-D.; Nassenstein, C.; Müller, M.; Ebensen, T.; Dittrich, A.-M.; et al. A Toll-Like Receptor 2/6 Agonist Reduces Allergic Airway Inflammation in Chronic Respiratory Sensitisation to Timothy Grass Pollen Antigens. Int. Arch. Allergy Immunol. 2010, 152, 131–139. [Google Scholar] [CrossRef]
- Schülke, S.; Fiedler, A.-H.; Junker, A.-C.; Flaczyk, A.; Wolfheimer, S.; Wangorsch, A.; Heinz, A.; Beckert, H.; Nagl, B.; Bohle, B.; et al. Critical role of mammalian target of rapamycin for IL-10 dendritic cell induction by a flagellin A conjugate in preventing allergic sensitization. J. Allergy Clin. Immunol. 2018, 141, 1786–1798.e11. [Google Scholar] [CrossRef] [Green Version]
- Puggioni, F.; Durham, S.R.; Francis, J.N. Monophosphoryl lipid A (MPLR)* promotes allergen-induced immune deviation in favour of Th1 responses. Allergy 2005, 60, 678–684. [Google Scholar] [CrossRef]
- Pfaar, O.; Barth, C.; Jaschke, C.; Hormann, K.; Klimek, L. Sublingual Allergen-Specific Immunotherapy Adjuvanted with Monophosphoryl Lipid A: A Phase I/IIa Study. Int. Arch. Allergy Immunol. 2010, 154, 336–344. [Google Scholar] [CrossRef]
- Worm, M.; Ernst, D.; Kraller, M.; Babina, M. The Impact on Allergy-Related Cells of a Birch Pollen Allergoid, with and without Monophosphoryl Lipid A, in Comparison with the Native Equivalent. Int. Arch. Allergy Immunol. 2017, 172, 20–26. [Google Scholar] [CrossRef]
- Rosewich, M.; Lee, D.; Zielen, S. Pollinex Quattro: An innovative four injections immunotherapy In allergic rhinitis. Hum. Vaccines Immunother. 2013, 9, 1523–1531. [Google Scholar] [CrossRef] [Green Version]
- Rosewich, M.; Girod, K.; Zielen, S.; Schubert, R.; Schulze, J. Induction of Bronchial Tolerance After 1 Cycle of Monophosphoryl-A-Adjuvanted Specific Immunotherapy in Children With Grass Pollen Allergies. Allergy Asthma Immunol. Res. 2016, 8, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Crivellaro, M.A.; Senna, G.E.; Pappacoda, A.; Vanzelli, R.; Spacal, B.; Marchi, G.; Recchia, G.; Makatsori, M. Safety of ultrashort-term sit with pollen allergoids adjuvanted by monophosphoryl lipid A: A prospective Italian survey. Eur. Ann. Allergy Clin. Immunol. 2011, 43, 58–60. [Google Scholar]
- Worm, M.; Higenbottam, T.; Pfaar, O.; Mösges, R.; Aberer, W.; Gunawardena, K.; Wessiepe, D.; Lee, D.; Kramer, M.; Skinner, M.; et al. Randomized controlled trials define shape of dose response for Pollinex Quattro Birch allergoid immunotherapy. Allergy 2018, 73, 1812–1822. [Google Scholar] [CrossRef]
- Musarra, A.; Bignardi, D.; Troise, C.; Passalacqua, G. Long-lasting effect of a monophosphoryl lipid-adjuvanted immunotherapy to parietaria. A controlled field study. Eur. Ann. Allergy Clin. Immunol. 2010, 42, 115–119. [Google Scholar]
- Zielen, S.; Gabrielpillai, J.; Herrmann, E.; Schulze, J.; Schubert, R.; Rosewich, M. Long-term effect of monophosphoryl lipid A adjuvanted specific immunotherapy in patients with grass pollen allergy. Immunotherapy 2018, 10, 529–536. [Google Scholar] [CrossRef]
- Farrokhi, S.; Mousavi, T.; Arshi, S.; Varasteh, A.; Rezaei, N.; Salekmoghadam, A. Co-Administration of Chenopodium Album Allergens and CpG Oligodeoxy-nucleotides Effects on Peripheral Blood Mononuclear Cells of Patients with Allergic Rhinitis Treated with Intranasal Corticosteroids. Iran J. Allergy Asthma Immunol. 2011, 10, 101–110. [Google Scholar]
- Kubo, S.; Yamada, T.; Osawa, Y.; Ito, Y.; Narita, N.; Fujieda, S. Cytosine–phosphate–guanosine-DNA induces CD274 expression in human B cells and suppresses T helper type 2 cytokine production in pollen antigen-stimulated CD4-positive cells. Clin. Exp. Immunol. 2012, 169, 1–9. [Google Scholar] [CrossRef]
- Leaker, B.R.; Singh, D.; Lindgren, S.; Almqvist, G.; Eriksson, L.; Young, B.; O’Connor, B. Effects of the Toll-like receptor 7 (TLR7) agonist, AZD8848, on allergen-induced responses in patients with mild asthma: A double-blind, randomised, parallel-group study. Respir. Res. 2019, 20, 1–11. [Google Scholar] [CrossRef]
- Prangtaworn, P.; Chaisri, U.; Seesuay, W.; Mahasongkram, K.; Onlamoon, N.; Reamtong, O.; Tungtrongchitr, A.; Indrawattana, N.; Chaicumpa, W.; Sookrung, N. Tregitope-linked Refined Allergen Vaccines for Immunotherapy in Cockroach Allergy. Sci. Rep. 2018, 8, 15480. [Google Scholar] [CrossRef]
- Foged, C.; Arigita, C.; Sundblad, A.; Jiskoot, W.; Storm, G.; Frokjaer, S. Interaction of dendritic cells with antigen-containing liposomes: Effect of bilayer composition. Vaccine 2004, 22, 1903–1913. [Google Scholar] [CrossRef]
- Meechan, P.; Tungtrongchitr, A.; Chaisri, U.; Maklon, K.; Indrawattana, N.; Chaicumpa, W.; Sookrung, N. Intranasal, Liposome-Adjuvanted Cockroach Allergy Vaccines Made of Refined Major Allergen and Whole-Body Extract of Periplaneta americana. Int. Arch. Allergy Immunol. 2013, 161, 351–362. [Google Scholar] [CrossRef]
- Kawakita, A.; Shirasaki, H.; Yasutomi, M.; Tokuriki, S.; Mayumi, M.; Naiki, H.; Ohshima, Y. Immunotherapy with oligomannose-coated liposomes ameliorates allergic symptoms in a murine food allergy model. Allergy 2012, 67, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Basomba, A.; Tabar, A.I.; de Rojas, D.H.F.; García, B.E.; Alamar, R.; Olaguíbel, J.M.; del Prado, J.M.; Martín, S.; Rico, P. Allergen vaccination with a liposome-encapsulated extract of Dermatophagoides pteronyssinus: A randomized, double-blind, placebo-controlled trial in asthmatic patients. J. Allergy Clin. Immunol. 2002, 109, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.J.; Echechipía, S.; Garcia, B.; Tabar, A.I.; Martin, S.; Rico, P.; Olaguibel, J.M. Liposome-entrappedD. pteronyssinusvaccination in mild asthma patients: Effect of 1-year double-blind, placebo-controlled trial on inflammation, bronchial hyper-responsiveness and immediate and late bronchial responses to the allergen. Clin. Exp. Allergy 2002, 32, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Schmidt-Weber, C.B.; Kramer, M.F.; Skinner, M.A.; Heath, M.D. Clinical use of adjuvants in allergen-immunotherapy. Expert Rev. Clin. Immunol. 2017, 13, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoms, F.; Jennings, G.T.; Maudrich, M.; Vogel, M.; Haas, S.; Zeltins, A.; Hofmann-Lehmann, R.; Riond, B.; Grossmann, J.; Hunziker, P.; et al. Immunization of cats to induce neutralizing antibodies against Fel d 1, the major feline allergen in human subjects. J. Allergy Clin. Immunol. 2019, 144, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimek, L.; Willers, J.; Hammann-Haenni, A.; Pfaar, O.; Stocker, H.; Mueller, P.; Renner, W.A.; Bachmann, M.F. Assessment of clinical efficacy of CYT003-QbG10 in patients with allergic rhinoconjunctivitis: A phase IIb study. Clin. Exp. Allergy 2011, 41, 1305–1312. [Google Scholar] [CrossRef]
- Beeh, K.-M.; Kanniess, F.; Wagner, F.; Schilder, C.; Naudts, I.; Hammann-Haenni, A.; Willers, J.; Stocker, H.; Mueller, P.; Bachmann, M.F.; et al. The novel TLR-9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. J. Allergy Clin. Immunol. 2013, 131, 866–874. [Google Scholar] [CrossRef]
- Jensen-Jarolim, E.; Roth-Walter, F.; Jordakieva, G.; Pali-Schöll, I. Allergens and Adjuvants in Allergen Immunotherapy for Immune Activation, Tolerance, and Resilience. J. Allergy Clin. Immunol. Pract. 2021, 9, 1780–1789. [Google Scholar] [CrossRef]
- Feng, Z.; Yi, X.; Hajavi, J. New and old adjuvants in allergen-specific immunotherapy: With a focus on nanoparticles. J. Cell. Physiol. 2021, 236, 863–876. [Google Scholar] [CrossRef]
- Zhao, H.; Verma, D.; Li, W.; Choi, Y.; Ndong, C.; Fiering, S.N.; Bailey-Kellogg, C.; Griswold, K.E. Depletion of T Cell Epitopes in Lysostaphin Mitigates Anti-Drug Antibody Response and Enhances Antibacterial Efficacy In Vivo. Chem. Biol. 2015, 22, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Norman, P.S.; Ohman, J.L.; Long, A.A.; Creticos, P.S.; Gefter, M.A.; Shaked, Z.; Wood, R.A.; Eggleston, P.A.; Hafner, K.B.; Rao, P.; et al. Treatment of cat allergy with T-cell reactive peptides. Am. J. Respir. Crit. Care Med. 1996, 154, 1623–1628. [Google Scholar] [CrossRef]
- Spertini, F.; Perrin, Y.; Audran, R.; Pellaton, C.; Boudousquié, C.; Barbier, N.; Thierry, A.-C.; Charlon, V.; Reymond, C. Safety and immunogenicity of immunotherapy with Bet v 1–derived contiguous overlapping peptides. J. Allergy Clin. Immunol. 2014, 134, 239–240.e13. [Google Scholar] [CrossRef]
- Gunawardana, N.C.; Durham, S.R. New approaches to allergen immunotherapy. Ann. Allergy Asthma Immunol. 2018, 121, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Eckl-Dorna, J.; Weber, M.; Stanek, V.; Linhart, B.; Ristl, R.; Waltl, E.E.; Merino, S.V.; Hummel, A.; Focke-Tejkl, M.; Froeschel, R.; et al. Two years of treatment with the recombinant grass pollen allergy vaccine BM32 induces a continuously increasing allergen-specific IgG4 response. EBioMedicine 2019, 50, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Tscheppe, A.; Breiteneder, H. Recombinant Allergens in Structural Biology, Diagnosis, and Immunotherapy. Int. Arch. Allergy Immunol. 2017, 172, 187–202. [Google Scholar] [CrossRef]
- Raith, M.; Zach, D.; Sonnleitner, L.; Woroszylo, K.; Focke-Tejkl, M.; Wank, H.; Graf, T.; Kuehn, A.; Pascal, M.; Muñoz-Cano, R.M.; et al. Rational design of a hypoallergenic Phl p 7 variant for immunotherapy of polcalcin-sensitized patients. Sci. Rep. 2019, 9, 7802. [Google Scholar] [CrossRef]
- Sancho, A.I.; Wallner, M.; Hauser, M.; Nagl, B.; Himly, M.; Asam, C.; Ebner, C.; Jahn-Schmid, B.; Bohle, B.; Ferreira, F. T Cell Epitope-Containing Domains of Ragweed Amb a 1 and Mugwort Art v 6 Modulate Immunologic Responses in Humans and Mice. PLoS ONE 2017, 12, e0169784. [Google Scholar] [CrossRef] [Green Version]
- Schülke, S.; Kuttich, K.; Wolfheimer, S.; Duschek, N.; Wangorsch, A.; Reuter, A.; Briza, P.; Pablos, I.; Gadermaier, G.; Ferreira, F.; et al. Conjugation of wildtype and hypoallergenic mugwort allergen Art v 1 to flagellin induces IL-10-DC and suppresses allergen-specific TH2-responses in vivo. Sci. Rep. 2017, 7, 11782. [Google Scholar] [CrossRef] [Green Version]
- De Groot, A.S.; Moise, L.; Terry, F.; Gutierrez, A.; Hindocha, P.; Richard, G.; Hoft, D.F.; Ross, T.M.; Noe, A.R.; Takahashi, Y.; et al. Better Epitope Discovery, Precision Immune Engineering, and Accelerated Vaccine Design Using Immunoinformatics Tools. Front. Immunol. 2020, 11, 442. [Google Scholar] [CrossRef]
- Martinez, D.; Cantillo, J.F.; Herazo, H.; Wortmann, J.; Keller, W.; Caraballo, L.; Puerta, L. Characterization of a hybrid protein designed with segments of allergens from Blomia tropicalis and Dermatophagoides pteronyssinus. Immunol. Lett. 2018, 196, 103–112. [Google Scholar] [CrossRef]
- Da Silva, E.S.; Aglas, L.; Pinheiro, C.S.; Belitardo, E.M.M.D.A.; Silveira, E.F.; Huber, S.; Torres, R.T.; Wallner, M.; Briza, P.; Lackner, P.; et al. A hybrid of two major Blomia tropicalis allergens as an allergy vaccine candidate. Clin. Exp. Allergy 2020, 50, 835–847. [Google Scholar] [CrossRef]
- Martínez, D.; Munera, M.; Cantillo, J.F.; Wortmann, J.; Zakzuk, J.; Keller, W.; Caraballo, L.; Puerta, L. An Engineered Hybrid Protein from Dermatophagoides pteronyssinus Allergens Shows Hypoallergenicity. Int. J. Mol. Sci. 2019, 20, 3025. [Google Scholar] [CrossRef] [Green Version]
- Mothes, N.; Heinzkill, M.; Drachenberg, K.J.; Sperr, W.R.; Krauth, M.T.; Majlesi, Y.; Semper, H.; Valent, P.; Niederberger, V.; Kraft, D.; et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: Reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin. Exp. Allergy 2003, 33, 1198–1208. [Google Scholar] [CrossRef]
- Jutel, M.; Jaeger, L.; Suck, R.; Meyer, H.; Fiebig, H.; Cromwell, O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J. Allergy Clin. Immunol. 2005, 116, 608–613. [Google Scholar] [CrossRef]
- Saarne, T.; Neimert-Andersson, T.; Grönlund, H.; Jutel, M.; Gafvelin, G.; van Hage, M. Treatment with a Fel d 1 hypoallergen reduces allergic responses in a mouse model for cat allergy. Allergy 2010, 66, 255–263. [Google Scholar] [CrossRef]
- Klimek, L.; Pfaar, O.; Worm, M. New opportunities for allergen immunotherapy using synthetic peptide immuno-regulatory epitopes (SPIREs). Expert Rev. Clin. Immunol. 2016, 12, 1123–1135. [Google Scholar] [CrossRef]
- Maguirea, P.; Nicodemusb, C.; Robinsonb, D.; Aaronson, D.; Umetsu, D.T. The Safety and Efficacy of ALLERVAX CAT in Cat Allergic Patients. Clin. Immunol. 1999, 93, 222–231. [Google Scholar] [CrossRef]
- Worm, M.; Lee, H.-H.; Kleine-Tebbe, J.; Hafner, R.P.; Laidler, P.; Healey, D.; Buhot, C.; Verhoef, A.; Maillère, B.; Kay, A.B.; et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J. Allergy Clin. Immunol. 2011, 127, 89–97.e14. [Google Scholar] [CrossRef]
- Couroux, P.; Patel, D.; Hafner, R.P.; Armstrong, K.; Larche, M. Fel d 1-derived synthetic peptide immuno-regulatory epitopes show a long-term treatment effect in cat allergic subjects. Clin. Exp. Allergy 2015, 45, 974–981. [Google Scholar] [CrossRef] [Green Version]
- Rudulier, C.D.; Tonti, E.; James, E.; Kwok, W.W.; Larché, M. Modulation of CRTh2 expression on allergen-specific T cells following peptide immunotherapy. Allergy 2019, 74, 2157–2166. [Google Scholar] [CrossRef] [Green Version]
- Senti, G.; Crameri, R.; Kuster, D.; Johansen, P.; Martinez-Gomez, J.M.; Graf, N.; Steiner, M.; Hothorn, L.A.; Grönlund, H.; Tivig, C.; et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J. Allergy Clin. Immunol. 2012, 129, 1290–1296. [Google Scholar] [CrossRef]
- Grönlund, H.; Gafvelin, G. Recombinant Bet v 1 vaccine for treatment of allergy to birch pollen. Hum. Vaccines 2010, 6, 970–977. [Google Scholar] [CrossRef]
- Niederberger, V.; Horak, F.; Vrtala, S.; Spitzauer, S.; Krauth, M.-T.; Valent, P.; Reisinger, J.; Pelzmann, M.; Hayek, B.; Kronqvist, M.; et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc. Natl. Acad. Sci. USA 2004, 101, 14677–14682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisinger, J.; Horak, F.; Pauli, G.; van Hage, M.; Cromwell, O.; König, F.; Valenta, R.; Niederberger, V. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J. Allergy Clin. Immunol. 2005, 116, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Gafvelin, G.; Thunberg, S.; Kronqvist, M.; Grönlund, H.; Grönneberg, R.; Troye-Blomberg, M.; Akdis, M.; Fiebig, H.; Purohit, A.; Horak, F.; et al. Cytokine and Antibody Responses in Birch-Pollen-Allergic Patients Treated with Genetically Modified Derivatives of the Major Birch Pollen Allergen Bet v 1. Int. Arch. Allergy Immunol. 2005, 138, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Moritz, K.; Neubauer, A.; Huber, H.; Henning, R.; Brodie, T.M.; Kaider, A.; Sallusto, F.; Wöhrl, S.; Valenta, R. Epicutaneous allergen application preferentially boosts specific T cell responses in sensitized patients. Sci. Rep. 2017, 7, 11657. [Google Scholar] [CrossRef] [Green Version]
- Meyer, W.; Narkus, A.; Salapatek, A.M.; Hafner, D. Double-blind, placebo-controlled, dose-ranging study of new recombinant hypoallergenic Bet v 1 in an environmental exposure chamber. Allergy 2013, 68, 724–731. [Google Scholar] [CrossRef]
- Nony, E.; Bouley, J.; Le Mignon, M.; Lemoine, P.; Jain, K.; Horiot, S.; Mascarell, L.; Pallardy, M.; Vincentelli, R.; Leone, P.; et al. Development and evaluation of a sublingual tablet based on recombinant Bet v 1 in birch pollen-allergic patients. Allergy 2015, 70, 795–804. [Google Scholar] [CrossRef]
- Gehlhar, K.; Schlaak, M.; Becker, W.-M.; Bufe, A. Monitoring allergen immunotherapy of pollen-allergic patients: The ratio of allergen-specific IgG4 to IgG1 correlates with clinical outcome. Clin. Exp. Allergy 1999, 29, 497–506. [Google Scholar] [CrossRef]
- Cornelius, C.; Schöneweis, K.; Georgi, F.; Weber, M.; Niederberger, V.; Zieglmayer, P.; Niespodziana, K.; Trauner, M.; Hofer, H.; Urban, S.; et al. Immunotherapy With the PreS-based Grass Pollen Allergy Vaccine BM32 Induces Antibody Responses Protecting Against Hepatitis B Infection. EBioMedicine 2016, 11, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Valenta, R.; Campana, R.; Niederberger, V. Recombinant allergy vaccines based on allergen-derived B cell epitopes. Immunol. Lett. 2017, 189, 19–26. [Google Scholar] [CrossRef]
- Niederberger, V.; Neubauer, A.; Gevaert, P.; Zidarn, M.; Worm, M.; Aberer, W.; Malling, H.J.; Pfaar, O.; Klimek, L.; Pfützner, W.; et al. Safety and efficacy of immunotherapy with the recombinant B-cell epitope–based grass pollen vaccine BM32. J. Allergy Clin. Immunol. 2018, 142, 497–509.e9. [Google Scholar] [CrossRef] [Green Version]
- Zieglmayer, P.; Focke-Tejkl, M.; Schmutz, R.; Lemell, P.; Zieglmayer, R.; Weber, M.; Kiss, R.; Blatt, K.; Valent, P.; Stolz, F.; et al. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EBioMedicine 2016, 11, 43–57. [Google Scholar] [CrossRef] [Green Version]
- Rauber, M.M.; Möbs, C.; Campana, R.; Henning, R.; Schulze-Dasbeck, M.; Greene, B.; Focke-Tejkl, M.; Weber, M.; Valenta, R.; Pfützner, W. Allergen immunotherapy with the hypoallergenic B-cell epitope-based vaccine BM32 modifies IL-10- and IL-5-secreting T cells. Allergy 2020, 75, 450–453. [Google Scholar] [CrossRef]
- Cooke, R.A.; Barnard, J.H.; Hebald, S.; Stull, A. Serological evidence of immunity with coexisting sensitization in a type of human allergy (hay fever). J. Exp. Med. 1935, 62, 733. [Google Scholar] [CrossRef] [Green Version]
- Freidl, R.; Gstoettner, A.; Baranyi, U.; Swoboda, I.; Stolz, F.; Focke-Tejkl, M.; Wekerle, T.; van Ree, R.; Valenta, R.; Linhart, B. Blocking antibodies induced by immunization with a hypoallergenic parvalbumin mutant reduce allergic symptoms in a mouse model of fish allergy. J. Allergy Clin. Immunol. 2017, 139, 1897–1905.e1. [Google Scholar] [CrossRef] [Green Version]
- Storni, F.; Zeltins, A.; Balke, I.; Heath, M.D.; Kramer, M.F.; Skinner, M.A.; Zha, L.; Roesti, E.; Engeroff, P.; Muri, L.; et al. Vaccine against peanut allergy based on engineered virus-like particles displaying single major peanut allergens. J. Allergy Clin. Immunol. 2020, 145, 1240–1253.e3. [Google Scholar] [CrossRef]
- Orengo, J.M.; Radin, A.R.; Kamat, V.; Badithe, A.; Ben, L.H.; Bennett, B.L.; Zhong, S.; Birchard, D.; Limnander, A.; Rafique, A.; et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Wickman, M.; Lupinek, C.; Andersson, N.; Belgrave, D.; Asarnoj, A.; Benet, M.; Pinart, M.; Wieser, S.; Garcia-Aymerich, J.; Baar, A.; et al. Detection of IgE Reactivity to a Handful of Allergen Molecules in Early Childhood Predicts Respiratory Allergy in Adolescence. EBioMedicine 2017, 26, 91–99. [Google Scholar] [CrossRef]
- Campana, R.; Marth, K.; Zieglmayer, P.; Weber, M.; Lupinek, C.; Zhernov, Y.; Elisyutina, O.; Khaitov, M.; Rigler, E.; Westritschnig, K.; et al. Vaccination of nonallergic individuals with recombinant hypoallergenic fragments of birch pollen allergen Bet v 1: Safety, effects, and mechanisms. J. Allergy Clin. Immunol. 2019, 143, 1258–1261. [Google Scholar] [CrossRef] [Green Version]
- Geroldinger-Simic, M.; Zelniker, T.; Aberer, W.; Ebner, C.; Egger, C.; Greiderer, A.; Prem, N.; Lidholm, J.; Ballmer-Weber, B.K.; Vieths, S.; et al. Birch pollen–related food allergy: Clinical aspects and the role of allergen-specific IgE and IgG4 antibodies. J. Allergy Clin. Immunol. 2011, 127, 616–622.e1. [Google Scholar] [CrossRef]
- De Silva, D.; Geromi, M.; Panesar, S.S.; Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Cardona, V.; Dubois, A.E.J.; Halken, S.; et al. Acute and long-term management of food allergy: Systematic review. Allergy 2013, 69, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Treudler, R.; Franke, A.; Schmiedeknecht, A.; Ballmer-Weber, B.; Worm, M.; Werfel, T.; Jappe, U.; Biedermann, T.; Schmitt, J.; Brehler, R.; et al. BASALIT trial: Double-blind placebo-controlled allergen immunotherapy with rBet v 1-FV in birch-related soya allergy. Allergy 2017, 72, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Kinaciyan, T.; Nagl, B.; Faustmann, S.; Frommlet, F.; Kopp, S.; Wolkersdorfer, M.; Wöhrl, S.; Bastl, K.; Huber, H.; Berger, U.; et al. Efficacy and safety of 4 months of sublingual immunotherapy with recombinant Mal d 1 and Bet v 1 in patients with birch pollen–related apple allergy. J. Allergy Clin. Immunol. 2018, 141, 1002–1008. [Google Scholar] [CrossRef] [Green Version]
- Grilo, J.R.; Kitzmüller, C.; Aglas, L.; Acosta, G.S.; Vollmann, U.; Ebner, C.; Horak, F.; Kinaciyan, T.; Radauer, C.; Ferreira, F.; et al. IgE-cross-blocking antibodies to Fagales following sublingual immunotherapy with recombinant Bet v 1. Allergy 2021, 76, 2555–2564. [Google Scholar] [CrossRef]
- Tulaeva, I.; Cornelius, C.; Zieglmayer, P.; Zieglmayer, R.; Schmutz, R.; Lemell, P.; Weber, M.; Focke-Tejkl, M.; Karaulov, A.; Henning, R.; et al. Quantification, epitope mapping and genotype cross-reactivity of hepatitis B preS-specific antibodies in subjects vaccinated with different dosage regimens of BM32. EBioMedicine 2020, 59, 102953. [Google Scholar] [CrossRef]
- Zhernov, Y.; Curin, M.; Khaitov, M.; Karaulov, A.; Valenta, R. Recombinant allergens for immunotherapy: State of the art. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 402–414. [Google Scholar] [CrossRef]
- Su, Y.; Romeu-Bonilla, E.; Anagnostou, A.; Fitz-Patrick, D.; Hearl, W.; Heiland, T. Safety and long-term immunological effects of CryJ2-LAMP plasmid vaccine in Japanese red cedar atopic subjects: A phase I study. Hum. Vaccines Immunother. 2017, 13, 2804–2813. [Google Scholar] [CrossRef] [Green Version]
- Slater, J.E.; Paupore, E.; Zhang, Y.T.; Colberg-Poley, A.M. The latex allergen Hev b 5 transcript is widely distributed after subcutaneous injection in BALB/c mice of its DNA vaccine. J. Allergy Clin. Immunol. 1998, 102, 469–475. [Google Scholar] [CrossRef]
- Ibáñez, M.S.; Sastre, J. Molecular allergy diagnosis for the clinical characterization of asthma. Expert Rev. Mol. Diagn. 2015, 15, 789–799. [Google Scholar] [CrossRef]
- Thomas, W.R.; Stewart, G.A.; Simpson, R.; Chua, K.Y.; Plozza, T.M.; Dilworth, R.J.; Nisbet, A.; Turner, K.J. Cloning and Expression of DNA Coding for the Major House Dust Mite Allergen Der p 1 in Escherichia coli. Int. Arch. Allergy Immunol. 1988, 85, 127–129. [Google Scholar] [CrossRef]
- Sastre, J.; Ibáñez, M.S. Molecular diagnosis and immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 565–570. [Google Scholar] [CrossRef]
- Canonica, G.W.; Ansotegui, I.J.; Pawankar, R.; Schmid-Grendelmeier, P.; van Hage, M.; E Baena-Cagnani, C.; Melioli, G.; Nunes, C.; Passalacqua, G.; Rosenwasser, L.; et al. A WAO—ARIA—GA2LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ. J. 2013, 6, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuidmeer-Jongejan, L.; Huber, H.; Swoboda, I.; Rigby, N.; Versteeg, S.A.; Jensen, B.M.; Quaak, S.; Akkerdaas, J.H.; Blom, L.; Asturias, J.; et al. Development of a Hypoallergenic Recombinant Parvalbumin for First-in-Man Subcutaneous Immunotherapy of Fish Allergy. Int. Arch. Allergy Immunol. 2015, 166, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27 (Suppl. S2), 1–250. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Laipold, K.; Arzt-Gradwohl, L.; Čerpes, U.; Sturm, E.M.; Aberer, W.; Sturm, G.J. IgE multiplex testing in house dust mite allergy is utile, and sensitivity is comparable to extract-based singleplex testing. Allergy 2020, 75, 2091–2094. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Tebbe, J.; Jappe, U. Molecular allergy diagnostic tests: Development and relevance in clinical practice. Allergol. Sel. 2017, 1, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Armentia, A.; Herrero, M.; Martín-Armentia, B.; Rihs, H.-P.; Postigo, I.; Martínez-Quesada, J. Molecular diagnosis in cannabis allergy. J. Allergy Clin. Immunol. Pract. 2014, 2, 351–352. [Google Scholar] [CrossRef]
- Huerta-Ocampo, J.Á.; Valenzuela-Corral, A.; Robles-Burgueño, M.D.R.; Guzmán-Partida, A.M.; Oñate, M.; Ángel, H.; Vázquez-Moreno, L.; Pavón-Romero, G.F.; Terán, L.M. Proteomic identification of allergenic proteins in red oak (Quercus rubra) pollen. World Allergy Organ. J. 2020, 13, 100111. [Google Scholar] [CrossRef]
- Mani, B.M.; Huerta-Ocampo, J.A.; Garcia-Sanchez, J.R.; Barrera-Pacheco, A.; de la Rosa, A.P.B.; Teran, L.M. Identification of Ligustrum lucidum pollen allergens using a proteomics approach. Biochem. Biophys. Res. Commun. 2015, 468, 788–792. [Google Scholar] [CrossRef]
- Morales-Amparano, M.B.; Valenzuela-Corral, A.; Montfort, G.R.-C.; Vázquez-Moreno, L.; Escobedo-Moratilla, A.; Pastor-Palacios, G.; Ovando-Vázquez, C.; Teran, L.M.; Huerta-Ocampo, J. Ángel Immunoproteomic identification of allergenic proteins in pecan (Carya illinoinensis) pollen. J. Proteom. 2021, 248, 104348. [Google Scholar] [CrossRef]
- Neethirajan, S.; Weng, X.; Tah, A.; Cordero, J.; Ragavan, K. Nano-biosensor platforms for detecting food allergens—New trends. Sens. Bio-Sens. Res. 2018, 18, 13–30. [Google Scholar] [CrossRef]
- Morales-Amparano, M.B.; Huerta-Ocampo, J.; Huerta-Ocampo, J.Á.; Pastor-Palacios, G.; Teran, L.M. The Role of Enolases in Allergic Disease. J. Allergy Clin. Immunol. Pract. 2021, 9, 3026–3032. [Google Scholar] [CrossRef]
- Martin, J.G.; Panariti, A. Fenotipos del asma, ¿son importantes? Arch. Bronconeumol. Engl. Ed. 2017, 53, 177–179. [Google Scholar] [CrossRef]



| Adjuvants | Hybrid Proteins | Recombinants |
|---|---|---|
| Aluminum | BTH2 (Blomia tropicalis) | MAT Fel d 1 (Cat) |
| Microcrystaline Tyrosine | DPx4 (Dermatophagoides pteronyssinus) | CatPAD (Cat) |
| Calcium Phosphate | MAVAC-BD-2 (Blomia tropicalis and Dermatophagoides sp.) | REGN1908 (Cat) |
| Toll-like Receptors | rBet v 1 FV (Birch) | |
| Liposomes | rBet v 1 (Birch) | |
| Virus Like Particles | BM32 (Grass) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavón-Romero, G.F.; Parra-Vargas, M.I.; Ramírez-Jiménez, F.; Melgoza-Ruiz, E.; Serrano-Pérez, N.H.; Teran, L.M. Allergen Immunotherapy: Current and Future Trends. Cells 2022, 11, 212. https://doi.org/10.3390/cells11020212
Pavón-Romero GF, Parra-Vargas MI, Ramírez-Jiménez F, Melgoza-Ruiz E, Serrano-Pérez NH, Teran LM. Allergen Immunotherapy: Current and Future Trends. Cells. 2022; 11(2):212. https://doi.org/10.3390/cells11020212
Chicago/Turabian StylePavón-Romero, Gandhi F., Maria Itzel Parra-Vargas, Fernando Ramírez-Jiménez, Esmeralda Melgoza-Ruiz, Nancy H. Serrano-Pérez, and Luis M. Teran. 2022. "Allergen Immunotherapy: Current and Future Trends" Cells 11, no. 2: 212. https://doi.org/10.3390/cells11020212
APA StylePavón-Romero, G. F., Parra-Vargas, M. I., Ramírez-Jiménez, F., Melgoza-Ruiz, E., Serrano-Pérez, N. H., & Teran, L. M. (2022). Allergen Immunotherapy: Current and Future Trends. Cells, 11(2), 212. https://doi.org/10.3390/cells11020212