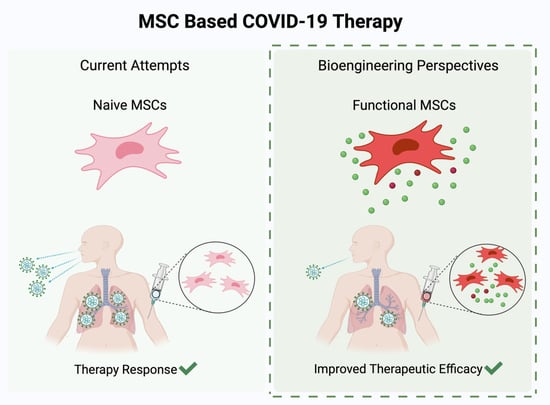
Mesenchymal Stem Cell-Based COVID-19 Therapy: Bioengineering Perspectives
Abstract
:1. Introduction
2. Mesenchymal Stem Cell Therapy
3. Bioengineering Solutions to Improve MSC Therapy
3.1. Tools for MSC Engineering
3.1.1. In Vitro Modeling
3.1.2. Assessing Efficacy in COVID-19 Animal Models
3.2. Clinical Route of Engineered MSCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- Fontanet, A.; Autran, B.; Lina, B.; Kieny, M.P.; Karim, S.S.A.; Sridhar, D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet 2021, 397, 952–954. [Google Scholar] [CrossRef]
- Abdool Karim, S.S.; de Oliveira, T. New SARS-CoV-2 Variants—Clinical, Public Health, and Vaccine Implications. N. Engl. J. Med. 2021, 384, 1866–1868. [Google Scholar] [CrossRef]
- Zhou, D.; Dejnirattisai, W.; Supasa, P.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Tuekprakhon, A.; Nutalai, R.; et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 2021, 184, 2348–2361.e6. [Google Scholar] [CrossRef]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020, 116, 1097–1100. [Google Scholar] [CrossRef] [Green Version]
- Lely, A.T.; Hamming, I.; van Goor, H.; Navis, G.J. Renal ACE2 expression in human kidney disease. J. Pathol. 2004, 204, 587–593. [Google Scholar] [CrossRef]
- Antinori, S.; Cossu, M.V.; Ridolfo, A.L.; Rech, R.; Bonazzetti, C.; Pagani, G.; Gubertini, G.; Coen, M.; Magni, C.; Castelli, A.; et al. Compassionate remdesivir treatment of severe COVID-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: Clinical outcome and differences in post-treatment hospitalisation status. Pharmacol. Res. 2020, 158, 104899. [Google Scholar] [CrossRef]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19); StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ahn, D.-G.; Shin, H.-J.; Kim, M.-H.; Lee, S.; Kim, H.-S.; Myoung, J.; Kim, B.-T.; Kim, S.-J. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J. Microbiol. Biotechnol. 2020, 30, 313–324. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- Cortegiani, A.; Ingoglia, G.; Ippolito, M.; Giarratano, A.; Einav, S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 2020, 57, 279–283. [Google Scholar] [CrossRef]
- Saad, J.; Mathew, D. Nonsteroidal Anti-Inflammatory Drugs Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kumar, S.; Saurabh, M.; Maharshi, V. Efficacy and safety of potential vaccine candidates against coronavirus disease 2019: A systematic review. J. Adv. Pharm. Technol. Res. 2021, 12, 215–221. [Google Scholar] [CrossRef]
- Lv, M.; Luo, X.; Shen, Q.; Lei, R.; Liu, X.; Liu, E.; Li, Q.; Chen, Y. Safety, immunogenicity, and efficacy of COVID-19 vaccines in children and adolescents: A systematic review. Vaccines 2021, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of pfizer/BioNTech and moderna vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [CrossRef]
- McDonald, I.; Murray, S.M.; Reynolds, C.J.; Altmann, D.M.; Boyton, R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines 2021, 6, 1–14. [Google Scholar] [CrossRef]
- Stuart, A.S.V.; Shaw, R.H.; Liu, X.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): A single-blind, randomised, phase 2, non-inferiority trial. Lancet 12 2021. [Google Scholar] [CrossRef]
- Yao, H.; Lu, X.; Chen, Q.; Xu, K.; Chen, Y.; Cheng, L.; Liu, F.; Wu, Z.; Wu, H.; Jin, C.; et al. Patient-derived mutations impact pathogenicity of SARS-CoV-2. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Pachetti, M.; Marini, B.; Benedetti, F.; Giudici, F.; Mauro, E.; Storici, P.; Masciovecchio, C.; Angeletti, S.; Ciccozzi, M.; Gallo, R.C.; et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J. Transl. Med. 2020, 18, 179. [Google Scholar] [CrossRef] [Green Version]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2021, 22, 43–55. [Google Scholar] [CrossRef]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community Transmission and Viral Load Kinetics of SARS-CoV-2 Delta (B.1.617.2)Variant in Vaccinated and Unvaccinated Individuals. SSRN Electron. J. 2021, 10, S1473-3099(21)00648-4. [Google Scholar] [CrossRef]
- Walter, J.; Ware, L.B.; Matthay, M.A. Mesenchymal stem cells: Mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir. Med. 2014, 2, 1016–1026. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Liu, Y.; Liu, H.; Li, Y. Genetically modified mesenchymal stem cell therapy for acute respiratory distress syndrome. Stem Cell Res. Ther. 2019, 10, 386. [Google Scholar] [CrossRef] [Green Version]
- Xiao, K.; Hou, F.; Huang, X.; Li, B.; Qian, Z.R.; Xie, L. Mesenchymal stem cells: Current clinical progress in ARDS and COVID-19. Stem Cell Res. Ther. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Gorman, E.; Millar, J.; McAuley, D.; O’Kane, C. Mesenchymal stromal cells for acute respiratory distress syndrome (ARDS), sepsis, and COVID-19 infection: Optimizing the therapeutic potential. Expert Rev. Respir. Med. 2021, 15, 301–324. [Google Scholar] [CrossRef] [PubMed]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Niu, C.; Li, R.; Huang, T.; Wang, Y.; Huang, M.; Ji, N.; Zheng, Y.; Chen, X.; Shi, L.; et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 361. [Google Scholar] [CrossRef]
- Shi, L.; Huang, H.; Lu, X.; Yan, X.; Jiang, X.; Xu, R.; Wang, S.; Zhang, C.; Yuan, X.; Xu, Z.; et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 2021, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Xu, R.; Wang, S.; Xu, Z.; Zhang, C.; Li, Y.; Yang, T.; Shi, L.; Fu, J.; Jiang, T.; et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: A phase 1 clinical trial. Signal Transduct. Target. Ther. 2020, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, S.M.R.; Aliannejad, R.; Zarrabi, M.; Soleimani, M.; Vosough, M.; Hosseini, S.E.; Hossieni, H.; Keshel, S.H.; Naderpour, Z.; Hajizadeh-Saffar, E.; et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: A case series. Stem Cell Res. Ther. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Martin, B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef]
- Bittira, B.; Shum-Tim, D.; Al-Khaldi, A.; Chiu, R.C.-J. Mobilization and homing of bone marrow stromal cells in myocardial infarction. Eur. J. Cardiothorac. Surg. 2003, 24, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.D.; Nolta, J.A.; Jin, Y.-S.; Barr, M.L.; Yu, H.; Starnes, V.A.; Cramer, D.V. Migration of mesenchymal stem cells to heart allografts during chronic rejection. Transplantation 2003, 75, 679–685. [Google Scholar] [CrossRef]
- Hung, S.-C.; Pochampally, R.R.; Chen, S.-C.; Hsu, S.-C.; Prockop, D.J. Angiogenic Effects of Human Multipotent Stromal Cell Conditioned Medium Activate the PI3K-Akt Pathway in Hypoxic Endothelial Cells to Inhibit Apoptosis, Increase Survival, and Stimulate Angiogenesis. Stem Cells 2007, 25, 2363–2370. [Google Scholar] [CrossRef]
- Shyu, K.-G.; Wang, B.-W.; Hung, H.-F.; Chang, C.-C.; Shih, D.T.-B. Mesenchymal stem cells are superior to angiogenic growth factor genes for improving myocardial performance in the mouse model of acute myocardial infarction. J. Biomed. Sci. 2006, 13, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uccelli, A.; Moretta, L.; Pistoia, V. Immunoregulatory function of mesenchymal stem cells. Eur. J. Immunol. 2006, 36, 2566–2573. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Pistoia, V.; Moretta, L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007, 28, 219–226. [Google Scholar] [CrossRef]
- Sehmi, R.; Baatjes, A.J.; Denburg, J.A. Hemopoietic progenitor cells and hemopoietic factors: Potential targets for treatment of allergic inflammatory diseases. Curr. Drug Targets. Inflamm. Allergy 2003, 2, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Shuaib, A.; Motan, D.; Bhattacharya, P.; McNabb, A.; Skerry, T.M.; Lacroix, D. Heterogeneity in The Mechanical Properties of Integrins Determines Mechanotransduction Dynamics in Bone Osteoblasts. Sci. Rep. 2019, 9, 13113. [Google Scholar] [CrossRef] [PubMed]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J. Canadian Critical Care Trials Group Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef]
- Kimbrel, E.A.; Lanza, R. Next-generation stem cells—Ushering in a new era of cell-based therapies. Nat. Rev. Drug Discov. 2020, 19, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-S.; Hayashi, M.; Ito, H.; Furutani, A.; Murata, T.; Matsuzaki, M.; Hamano, K. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation 2005, 111, 2438–2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iso, Y.; Spees, J.L.; Serrano, C.; Bakondi, B.; Pochampally, R.; Song, Y.-H.; Sobel, B.E.; Delafontaine, P.; Prockop, D.J. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem. Biophys. Res. Commun. 2007, 354, 700–706. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.H.; Seo, M.J.; Reger, R.L.; Spees, J.L.; Pulin, A.A.; Olson, S.D.; Prockop, D.J. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc. Natl. Acad. Sci. USA 2006, 103, 17438–17443. [Google Scholar] [CrossRef] [Green Version]
- Veevers-Lowe, J.; Ball, S.G.; Shuttleworth, A.; Kielty, C.M. Mesenchymal stem cell migration is regulated by fibronectin through α5β1-integrin-mediated activation of PDGFR-β and potentiation of growth factor signals. J. Cell Sci. 2011, 124, 1288–1300. [Google Scholar] [CrossRef] [Green Version]
- Baron, F.; Lechanteur, C.; Willems, E.; Bruck, F.; Baudoux, E.; Seidel, L.; Vanbellinghen, J.-F.; Hafraoui, K.; Lejeune, M.; Gothot, A.; et al. Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol. Blood Marrow Transplant. 2010, 16, 838–847. [Google Scholar] [CrossRef] [Green Version]
- MacMillan, M.L.; Blazar, B.R.; DeFor, T.E.; Wagner, J.E. Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: Results of a phase I-II clinical trial. Bone Marrow Transplant. 2009, 43, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Keller, C.A.; Gonwa, T.A.; Hodge, D.O.; Hei, D.J.; Centanni, J.M.; Zubair, A.C. Feasibility, Safety, and Tolerance of Mesenchymal Stem Cell Therapy for Obstructive Chronic Lung Allograft Dysfunction. Stem Cells Transl. Med. 2018, 7, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Glassberg, M.K.; Minkiewicz, J.; Toonkel, R.L.; Simonet, E.S.; Rubio, G.A.; DiFede, D.; Shafazand, S.; Khan, A.; Pujol, M.V.; LaRussa, V.F.; et al. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest 2017, 151, 971–981. [Google Scholar] [CrossRef]
- Zheng, G.; Huang, L.; Tong, H.; Shu, Q.; Hu, Y.; Ge, M.; Deng, K.; Zhang, L.; Zou, B.; Cheng, B.; et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: A randomized, placebo-controlled pilot study. Respir. Res. 2014, 15, 39. [Google Scholar] [CrossRef] [Green Version]
- Bellingan, G.; Jacono, F.; Bannard-Smith, J.; Brealey, D.; Meyer, N.; Thickett, D.; Young, D.; Bentley, A.; McVerry, B.; Wunderink, R.G.; et al. Primary Analysis of a Phase 1/2 Study to Assess MultiStem® Cell Therapy, a Regenerative Advanced Therapy Medicinal Product (ATMP), in Acute Respiratory Distress Syndrome (MUST-ARDS). In B14. LATE BREAKING CLINICAL TRIALS; American Thoracic Society: Dallas, TX, USA, 2019; p. A7353. [Google Scholar]
- Yip, H.-K.; Fang, W.-F.; Li, Y.-C.; Lee, F.-Y.; Lee, C.-H.; Pei, S.-N.; Ma, M.-C.; Chen, K.-H.; Sung, P.-H.; Lee, M.S. Human Umbilical Cord-Derived Mesenchymal Stem Cells for Acute Respiratory Distress Syndrome. Crit. Care Med. 2020, 48, e391–e399. [Google Scholar] [CrossRef]
- Yamout, B.; Hourani, R.; Salti, H.; Barada, W.; El-Hajj, T.; Al-Kutoubi, A.; Herlopian, A.; Baz, E.K.; Mahfouz, R.; Khalil-Hamdan, R.; et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: A pilot study. J. Neuroimmunol. 2010, 227, 185–189. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Hua, B.; Wang, H.; Lu, L.; Shi, S.; Hou, Y.; Zeng, X.; Gilkeson, G.S.; Sun, L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann. Rheum. Dis. 2010, 69, 1423–1429. [Google Scholar] [CrossRef] [Green Version]
- García-Olmo, D.; García-Arranz, M.; Herreros, D.; Pascual, I.; Peiro, C.; Rodríguez-Montes, J.A. A phase I clinical trial of the treatment of crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis. Colon Rectum 2005, 48, 1416–1423. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Herreros, D.; Pascual, I.; Pascual, J.A.; Del-Valle, E.; Zorrilla, J.; De-La-Quintana, P.; Garcia-Arranz, M.; Pascual, M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis. Colon Rectum 2009, 52, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Atluri, S.; Manchikanti, L.; Hirsch, J.A. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician 2020, 23, E71–E83. [Google Scholar] [PubMed]
- Shinmura, D.; Togashi, I.; Miyoshi, S.; Nishiyama, N.; Hida, N.; Tsuji, H.; Tsuruta, H.; Segawa, K.; Tsukada, Y.; Ogawa, S.; et al. Pretreatment of human mesenchymal stem cells with pioglitazone improved efficiency of cardiomyogenic transdifferentiation and cardiac function. Stem Cells 2011, 29, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kim, Y.S.; Hong, S.H.; Oh, Y.M. Therapeutic effects of adipose-derived stem cells pretreated with pioglitazone in an emphysema mouse model. Exp. Mol. Med. 2016, 48, e266. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Choi, D.-Y.; Yun, S.J.; Choi, S.-M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.-W. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 2012, 11, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Lohan, P.; Treacy, O.; Griffin, M.D.; Ritter, T.; Ryan, A.E. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells and their extracellular vesicles: Are we still learning? Front. Immunol. 2017, 8, 1626. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.; Wang, Z.; Hare, J.M.; Bu, G.; Mallea, J.M.; Pascual, J.M.; Caplan, A.I.; Kurtzberg, J.; Zubair, A.C.; Kubrova, E.; et al. Cell-based therapy to reduce mortality from COVID-19: Systematic review and meta-analysis of human studies on acute respiratory distress syndrome. Stem Cells Transl. Med. 2020, 9, 1007–1022. [Google Scholar] [CrossRef]
- Avanzini, M.A.; Mura, M.; Percivalle, E.; Bastaroli, F.; Croce, S.; Valsecchi, C.; Lenta, E.; Nykjaer, G.; Cassaniti, I.; Bagnarino, J.; et al. Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl. Med. 2021, 10, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, H.; Zhai, W.; Wang, Y.; Li, M.; Li, M.; Yang, L.; Tian, Y.; Song, Y.; Li, J.; et al. A safety consideration of mesenchymal stem cell therapy on COVID-19. Stem Cell Res. 2020, 49, 102066. [Google Scholar] [CrossRef]
- Hernandez, J.J.; Beaty, D.E.; Fruhwirth, L.L.; Lopes Chaves, A.P.; Riordan, N.H. Dodging COVID-19 infection: Low expression and localization of ACE2 and TMPRSS2 in multiple donor-derived lines of human umbilical cord-derived mesenchymal stem cells. J. Transl. Med. 2021, 19, 149. [Google Scholar] [CrossRef]
- Desterke, C.; Griscelli, F.; Imeri, J.; Marcoux, P.; Lemonnier, T.; Latsis, T.; Turhan, A.G.; Bennaceur-Griscelli, A. Molecular investigation of adequate sources of mesenchymal stem cells for cell therapy of COVID-19-associated organ failure. Stem Cells Transl. Med. 2021, 10, 568–571. [Google Scholar] [CrossRef]
- Generali, M.; Kehl, D.; Wanner, D.; Okoniewski, M.J.; Hoerstrup, S.P.; Cinelli, P. Heterogeneous expression of ACE2 and TMPRRS2 in mesenchymal stromal cells. J. Cell. Mol. Med. 2021. [Google Scholar] [CrossRef]
- Barzegar, M.; Vital, S.; Stokes, K.Y.; Wang, Y.; Yun, J.W.; White, L.A.; Chernyshev, O.; Kelley, R.E.; Alexander, J.S. Human placenta mesenchymal stem cell protection in ischemic stroke is angiotensin converting enzyme-2 and masR receptor-dependent. Stem Cells 2021, 39, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Banu, N.; Panikar, S.S.; Leal, L.R.; Leal, A.R. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to Macrophage Activation Syndrome: Therapeutic implications. Life Sci. 2020, 256, 117905. [Google Scholar] [CrossRef]
- Shah, K. Mesenchymal stem cells engineered for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 739–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowakowski, A.; Drela, K.; Rozycka, J.; Janowski, M.; Lukomska, B. Engineered Mesenchymal Stem Cells as an Anti-Cancer Trojan Horse. Stem Cells Dev. 2016, 25, 1513–1531. [Google Scholar] [CrossRef]
- Deng, P.; Torrest, A.; Pollock, K.; Dahlenburg, H.; Annett, G.; Nolta, J.A.; Fink, K.D. Clinical trial perspective for adult and juvenile Huntington’s disease using genetically-engineered mesenchymal stem cells. Neural Regen. Res. 2016, 11, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lian, Y.; Deng, Y.; Chen, J.; Wu, T.; Lai, X.; Zheng, B.; Qiu, C.; Peng, Y.; Li, W.; et al. mRNA-engineered mesenchymal stromal cells expressing CXCR2 enhances cell migration and improves recovery in IBD. Mol. Ther.-Nucleic Acids 2021, 26, 226–236. [Google Scholar] [CrossRef]
- Kondo, M.; Kameishi, S.; Grainger, D.W.; Okano, T. Novel therapies using cell sheets engineered from allogeneic mesenchymal stem/stromal cells. Emerg. Top. Life Sci. 2021, 4, 677–689. [Google Scholar] [CrossRef]
- Keshavarz, M.; Ebrahimzadeh, M.S.; Miri, S.M.; Dianat-Moghadam, H.; Ghorbanhosseini, S.S.; Mohebbi, S.R.; Keyvani, H.; Ghaemi, A. Oncolytic Newcastle disease virus delivered by Mesenchymal stem cells-engineered system enhances the therapeutic effects altering tumor microenvironment. Virol. J. 2020, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef]
- Carretta, M.; de Boer, B.; Jaques, J.; Antonelli, A.; Horton, S.J.; Yuan, H.; de Bruijn, J.D.; Groen, R.W.J.; Vellenga, E.; Schuringa, J.J. Genetically engineered mesenchymal stromal cells produce IL-3 and TPO to further improve human scaffold-based xenograft models. Exp. Hematol. 2017, 51, 36–46. [Google Scholar] [CrossRef]
- Hombach, A.A.; Geumann, U.; Günther, C.; Hermann, F.G.; Abken, H. IL7-IL12 Engineered Mesenchymal Stem Cells (MSCs) Improve A CAR T Cell Attack Against Colorectal Cancer Cells. Cells 2020, 9, 873. [Google Scholar] [CrossRef] [Green Version]
- Hingtgen, S.D.; Kasmieh, R.; van de Water, J.; Weissleder, R.; Shah, K. A Novel Molecule Integrating Therapeutic and Diagnostic Activities Reveals Multiple Aspects of Stem Cell-based Therapy. Stem Cells 2010, 28, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, T.M.; Buchmeier, M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology 2001, 279, 371–374. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yang, C.; Xu, X.-F.; Xu, W.; Liu, S. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Rokni, M.; Hamblin, M.R.; Rezaei, N. Cytokines and COVID-19: Friends or foes? Hum. Vaccines Immunother. 2020, 16, 2363–2365. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.; Davidson, S.M.; Yellon, D.M. The cytokine storm of COVID-19: A spotlight on prevention and protection. Expert Opin. Ther. Targets 2020, 24, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Niu, S.; Guo, B.; Gao, T.; Wang, L.; Wang, Y.; Wang, L.; Tan, Y.; Wu, J.; Hao, J. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif. 2020, 53, e12939. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhao, A. Mesenchymal stem cell therapy for acute respiratory distress syndrome: From basic to clinics. Protein Cell 2020, 11, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, F.; Wang, H.; Niu, G.; Choi, K.Y.; Swierczewska, M.; Zhang, G.; Gao, H.; Wang, Z.; Zhu, L.; et al. Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials 2013, 34, 1772–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Suryaprakash, S.; Lao, Y.H.; Leong, K.W. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods 2015, 84, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.T.; Kim, H.; Yun, J.; Chung, J.S.; Kwon, S.M. Promising Therapeutic Strategies for Mesenchymal Stem Cell-Based Cardiovascular Regeneration: From Cell Priming to Tissue Engineering. Stem Cells Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef]
- Phan, J.; Kumar, P.; Hao, D.; Gao, K.; Farmer, D.; Wang, A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J. Extracell. Vesicles 2018, 7, 1522236. [Google Scholar] [CrossRef] [PubMed]
- Sebastiano, V.; Maeder, M.L.; Angstman, J.F.; Haddad, B.; Khayter, C.; Yeo, D.T.; Goodwin, M.J.; Hawkins, J.S.; Ramirez, C.L.; Batista, L.F.Z.; et al. In situ genetic correction of the sickle cell anemia mutation in human induced pluripotent stem cells using engineered zinc finger nucleases. Stem Cells 2011, 29, 1717–1726. [Google Scholar] [CrossRef] [Green Version]
- Hockemeyer, D.; Wang, H.; Kiani, S.; Lai, C.S.; Gao, Q.; Cassady, J.P.; Cost, G.J.; Zhang, L.; Santiago, Y.; Miller, J.C.; et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat. Biotechnol. 2011, 29, 731–734. [Google Scholar] [CrossRef] [Green Version]
- Golchin, A.; Shams, F.; Karami, F. Advancing mesenchymal stem cell therapy with CRISPR/Cas9 for clinical trial studies. Adv. Exp. Med. Biol. 2020, 1247, 89–100. [Google Scholar]
- Filho, D.M.; de Carvalho Ribeiro, P.; Oliveira, L.F.; dos Santos, A.L.R.T.; Parreira, R.C.; Pinto, M.C.X.; Resende, R.R. Enhancing the Therapeutic Potential of Mesenchymal Stem Cells with the CRISPR-Cas System. Stem Cell Rev. Rep. 2019, 15, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Kearns, N.A.; Maehr, R. Transcriptional regulation with CRISPR/cas9 effectors in mammalian cells. Methods Mol. Biol. 2016, 1358, 43–57. [Google Scholar] [PubMed]
- Zarogoulidis, P.; Darwiche, K.; Sakkas, A.; Yarmus, L.; Huang, H.; Li, Q.; Freitag, L.; Zarogoulidis, K.; Malecki, M. Suicide Gene Therapy for Cancer-Current Strategies. J. Genet. Syndr. Gene Ther. 2013, 4, 16849. [Google Scholar] [CrossRef]
- Malecki, M. Frontiers in Suicide Gene Therapy of Cancer. J. Genet. Syndr. Gene Ther. 2012, 3, e114. [Google Scholar] [CrossRef]
- Niess, H.; von Einem, J.C.; Thomas, M.N.; Michl, M.; Angele, M.K.; Huss, R.; Günther, C.; Nelson, P.J.; Bruns, C.J.; Heinemann, V. Treatment of advanced gastrointestinal tumors with genetically modified autologous mesenchymal stromal cells (TREAT-ME1): Study protocol of a phase I/II clinical trial. BMC Cancer 2015, 15, 237. [Google Scholar] [CrossRef] [Green Version]
- Freytag, S.O.; Stricker, H.; Lu, M.; Elshaikh, M.; Aref, I.; Pradhan, D.; Levin, K.; Kim, J.H.; Peabody, J.; Siddiqui, F.; et al. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 268–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossignoli, F.; Grisendi, G.; Spano, C.; Golinelli, G.; Recchia, A.; Rovesti, G.; Orsi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Inducible Caspase9-mediated suicide gene for MSC-based cancer gene therapy. Cancer Gene Ther. 2019, 26, 11–16. [Google Scholar] [CrossRef]
- Straathof, K.C.; Pulè, M.A.; Yotnda, P.; Dotti, G.; Vanin, E.F.; Brenner, M.K.; Heslop, H.E.; Spencer, D.M.; Rooney, C.M. An inducible caspase 9 safety switch for T-cell therapy. Blood 2005, 105, 4247–4254. [Google Scholar] [CrossRef]
- Yeung, M.L.; Teng, J.L.L.; Jia, L.; Zhang, C.; Huang, C.; Cai, J.P.; Zhou, R.; Chan, K.H.; Zhao, H.; Zhu, L.; et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell 2021, 184, 2212–2228.e12. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Fontela, C.; Dowling, W.E.; Funnell, S.G.P.; Gsell, P.S.; Riveros-Balta, A.X.; Albrecht, R.A.; Andersen, H.; Baric, R.S.; Carroll, M.W.; Cavaleri, M.; et al. Animal models for COVID-19. Nature 2020, 586, 509–515. [Google Scholar] [CrossRef]
- Dinnon, K.H.; Leist, S.R.; Schäfer, A.; Edwards, C.E.; Martinez, D.R.; Montgomery, S.A.; West, A.; Yount, B.L.; Hou, Y.J.; Adams, L.E.; et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 2020, 586, 560–566. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Takayama, K. In Vitro and Animal Models for SARS-CoV-2 research. Trends Pharmacol. Sci. 2020, 41, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.D.; Irving, A.; Montagutelli, X.; Tate, M.D.; Rudloff, I.; Nold, M.F.; Hansbro, N.G.; Kim, R.Y.; Donovan, C.; Liu, G.; et al. Animal and translational models of SARS-CoV-2 infection and COVID-19. Mucosal Immunol. 2020, 13, 877–891. [Google Scholar] [CrossRef]
- Shah, K.; Hingtgen, S.; Kasmieh, R.; Figueiredo, J.L.; Garcia-Garcia, E.; Martinez-Serrano, A.; Breakefield, X.; Weissleder, R. Bimodal Viral Vectors and In Vivo Imaging Reveal the Fate of Human Neural Stem Cells in Experimental Glioma Model. J. Neurosci. 2008, 28, 4406–4413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalimuthu, S.; Oh, J.M.; Gangadaran, P.; Zhu, L.; Lee, H.W.; Jeon, Y.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Genetically engineered suicide gene in mesenchymal stem cells using a Tet-On system for anaplastic thyroid cancer. PLoS ONE 2017, 12, e0181318. [Google Scholar] [CrossRef] [Green Version]
- Freitas, J.; Santos, S.G.; Gonçalves, R.M.; Teixeira, J.H.; Barbosa, M.A.; Almeida, M.I. Genetically Engineered-MSC Therapies for Non-unions, Delayed Unions and Critical-size Bone Defects. Int. J. Mol. Sci. 2019, 20, 3430. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Lim, H.; Lee, D.; Choi, S.J.; Oh, W.; Yang, Y.S.; Oh, J.S.; Hwang, H.H.; Jeon, H.B. Thrombospondin-1 secreted by human umbilical cord blood-derived mesenchymal stem cells rescues neurons from synaptic dysfunction in Alzheimer’s disease model. Sci. Rep. 2018, 8, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoban, D.B.; Howard, L.; Dowd, E. GDNF-secreting mesenchymal stem cells provide localized neuroprotection in an inflammation-driven rat model of Parkinson’s disease. Neuroscience 2015, 303, 402–411. [Google Scholar] [CrossRef]
- Liao, W.; Pham, V.; Liu, L.; Riazifar, M.; Pone, E.J.; Zhang, S.X.; Ma, F.; Lu, M.; Walsh, C.M.; Zhao, W. Mesenchymal stem cells engineered to express selectin ligands and IL-10 exert enhanced therapeutic efficacy in murine experimental autoimmune encephalomyelitis. Biomaterials 2016, 77, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, S.D.; Pollock, K.; Kambal, A.; Cary, W.; Mitchell, G.-M.; Tempkin, J.; Stewart, H.; McGee, J.; Bauer, G.; Kim, H.S.; et al. Genetically engineered mesenchymal stem cells as a proposed therapeutic for Huntington’s disease. Mol. Neurobiol. 2012, 45, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Yang, Z.; Suo, Y.; Chen, Q.; Wei, D.; Weng, X.; Gu, Z.; Wei, X. Systemically Infused Mesenchymal Stem Cells Show Different Homing Profiles in Healthy and Tumor Mouse Models. Stem Cells Transl. Med. 2017, 6, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.-C.; Yan, J.; Liu, G.-D.; Tan, X.-Y.; Weng, X.-F.; Wu, W.-Z.; Zhou, J.; Wei, X.-B. Real-time monitoring of rare circulating hepatocellular carcinoma cells in an orthotopic model by in vivo flow cytometry assesses resection on metastasis. Cancer Res. 2012, 72, 2683–2691. [Google Scholar] [CrossRef] [Green Version]
- Wolfs, E.; Holvoet, B.; Gijsbers, R.; Casteels, C.; Roberts, S.J.; Struys, T.; Maris, M.; Ibrahimi, A.; Debyser, Z.; Van Laere, K.; et al. Optimization of multimodal imaging of mesenchymal stem cells using the human sodium iodide symporter for PET and Cerenkov luminescence imaging. PLoS ONE 2014, 9, e94833. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, Y.; Li, M.; Feng, H.; Liu, Q.; Qin, C.; Zhang, Y.; Lan, X. Using tyrosinase as a tri-modality reporter gene to monitor transplanted stem cells in acute myocardial infarction. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Patrick, P.S.; Kolluri, K.K.; Zaw Thin, M.; Edwards, A.; Sage, E.K.; Sanderson, T.; Weil, B.D.; Dickson, J.C.; Lythgoe, M.F.; Lowdell, M.; et al. Lung delivery of MSCs expressing anti-cancer protein TRAIL visualised with 89Zr-oxine PET-CT. Stem Cell Res. Ther. 2020, 11, 256. [Google Scholar] [CrossRef]
- Bordi, L.; Sberna, G.; Lalle, E.; Piselli, P.; Colavita, F.; Nicastri, E.; Antinori, A.; Boumis, E.; Petrosillo, N.; Marchioni, L.; et al. Frequency and Duration of SARS-CoV-2 Shedding in Oral Fluid Samples Assessed by a Modified Commercial Rapid Molecular Assay. Viruses 2020, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.D.; Wilson, J.G.; Zhuo, H.; Caballero, L.; McMillan, M.L.; Fang, X.; Cosgrove, K.; Calfee, C.S.; Lee, J.-W.; Kangelaris, K.N.; et al. Design and implementation of the START (STem cells for ARDS Treatment) trial, a phase 1/2 trial of human mesenchymal stem/stromal cells for the treatment of moderate-severe acute respiratory distress syndrome. Ann. Intensive Care 2014, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.-W.; et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir. Med. 2015, 3, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef]
- Chen, J.; Hu, C.; Chen, L.; Tang, L.; Zhu, Y.; Xu, X.; Chen, L.; Gao, H.; Lu, X.; Yu, L.; et al. Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment. Engineering 2020, 6, 1153–1161. [Google Scholar] [CrossRef]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, B.; Chen, J.; Li, T.; Wu, H.; Yang, W.; Li, Y.; Li, J.; Yu, C.; Nie, F.; Ma, Z.; et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine 2020, 99, e21429. [Google Scholar] [CrossRef]
- Gorman, E.; Shankar-Hari, M.; Hopkins, P.; Tunnicliffe, W.S.; Perkins, G.D.; Silversides, J.; McGuigan, P.; Jackson, C.; Boyle, R.; McFerran, J.; et al. Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration in COVID-19 (REALIST-COVID-19): A structured summary of a study protocol for a randomised, controlled trial. Trials 2020, 21, 462. [Google Scholar] [CrossRef]
- Masterson, C.H.; Curley, G.F.; Laffey, J.G. Modulating the distribution and fate of exogenously delivered MSCs to enhance therapeutic potential: Knowns and unknowns. Intensive Care Med. Exp. 2019, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rezaie, J.; Nouri, M.; Panahi, Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. 2020, 85, 104422. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Moradinasab, S.; Pourbagheri-Sigaroodi, A.; Zafari, P.; Ghaffari, S.H.; Bashash, D. Mesenchymal stromal/stem cells (MSCs) and MSC-derived extracellular vesicles in COVID-19-induced ARDS: Mechanisms of action, research progress, challenges, and opportunities. Int. Immunopharmacol. 2021, 97, 107694. [Google Scholar] [CrossRef]
- Moll, G.; Drzeniek, N.; Kamhieh-Milz, J.; Geissler, S.; Volk, H.D.; Reinke, P. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Front. Immunol. 2020, 11, 1091. [Google Scholar] [CrossRef]
- Dlouhy, B.J.; Awe, O.; Rao, R.C.; Kirby, P.A.; Hitchon, P.W. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient. J. Neurosurg. Spine 2014, 21, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Ankrum, J. Cell therapies can bring insult to injury. Sci. Transl. Med. 2020, 12, eabb0792. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakaş, N.; Üçüncüoğlu, S.; Uludağ, D.; Karaoğlan, B.S.; Shah, K.; Öztürk, G. Mesenchymal Stem Cell-Based COVID-19 Therapy: Bioengineering Perspectives. Cells 2022, 11, 465. https://doi.org/10.3390/cells11030465
Karakaş N, Üçüncüoğlu S, Uludağ D, Karaoğlan BS, Shah K, Öztürk G. Mesenchymal Stem Cell-Based COVID-19 Therapy: Bioengineering Perspectives. Cells. 2022; 11(3):465. https://doi.org/10.3390/cells11030465
Chicago/Turabian StyleKarakaş, Nihal, Süleyman Üçüncüoğlu, Damla Uludağ, Birnur Sinem Karaoğlan, Khalid Shah, and Gürkan Öztürk. 2022. "Mesenchymal Stem Cell-Based COVID-19 Therapy: Bioengineering Perspectives" Cells 11, no. 3: 465. https://doi.org/10.3390/cells11030465
APA StyleKarakaş, N., Üçüncüoğlu, S., Uludağ, D., Karaoğlan, B. S., Shah, K., & Öztürk, G. (2022). Mesenchymal Stem Cell-Based COVID-19 Therapy: Bioengineering Perspectives. Cells, 11(3), 465. https://doi.org/10.3390/cells11030465