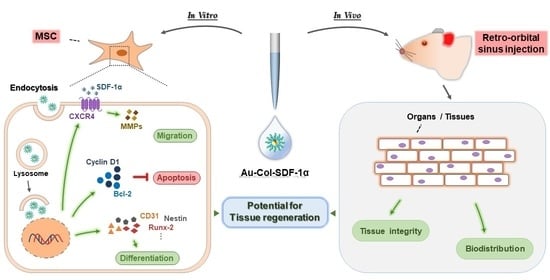
Differentiation Induction of Mesenchymal Stem Cells by a Au Delivery Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Au, Col and SDF-1α Materials
2.1.1. Au Nanoparticles (Au)
2.1.2. Stromal Cell-Derived Factor-1α (SDF-1α)
2.1.3. Type I Collagen (Col)
2.1.4. Au Combined with Collagen (Au-Col)
2.1.5. Au–Collagen Conjugated with Stromal Cell-Derived Factor-1α (Au-Col-SDF-1α)
2.2. Characterization of As-Prepared Nanomaterials
2.2.1. Ultraviolet–Visible Spectrophotometry (UV-Vis)
2.2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.3. Scanning Electron Microscopy (SEM)
2.2.4. Dynamic Light Scattering (DLS) Assay
2.3. Biocompatibility Examinations
2.3.1. Cell Culture
2.3.2. Cell Viability
2.3.3. Reactive Oxygen Species (ROS) Generation
2.4. Evaluation of Biological Performances
2.4.1. Matrix Metalloproteinases (MMPs) Activities
2.4.2. Migration Ability
2.4.3. Expression of CXCR4 and SDF-1α
2.5. Measurement of Cell Progression
2.5.1. Cell Cycle Analysis
2.5.2. Cell Apoptosis
2.5.3. Expression of Apoptotic Proteins via Western Blotting Assay
2.6. Determination of Differentiation Capacities
2.7. Real-Time Polymerase Chain Reaction (PCR)
2.8. Cell Uptake Mechanisms
2.8.1. Measurement of Cellular Uptake Efficiency and Mechanisms
2.8.2. LysoTracker Assay
2.9. In Vivo Biodistribution
2.10. Statistical Analysis
3. Results
3.1. Characterization of the Physicochemical Properties
3.2. Exploration of Biocompatibility and Biological Function
3.3. Analysis of Cell Cycle Progression and Apoptosis
3.4. Investigation of Differentiation Capacities
3.5. Examination of Cell Uptake Mechanisms
3.6. Assessments of Biodistribution in an Animal Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loizidou, M.; Seifalian, A. Nanotechnology and its applications in surgery. J. Br. Surg. 2010, 97, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Bolívar-Monsalve, E.J.; Alvarez, M.M.; Hosseini, S.; Espinosa-Hernandez, M.A.; Ceballos-González, C.F.; Sanchez-Dominguez, M.; Shin, S.R.; Cecen, B.; Hassan, S.; Di Maio, E. Engineering bioactive synthetic polymers for biomedical applications: A review with emphasis on tissue engineering and controlled release. Mater. Adv. 2021, 2, 4447–4478. [Google Scholar] [CrossRef]
- Bharathala, S.; Sharma, P. Biomedical applications of nanoparticles. In Nanotechnology in Modern Animal Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–132. [Google Scholar]
- Saji, V.S.; Choe, H.C.; Yeung, K.W. Nanotechnology in biomedical applications: A review. Int. J. Nano Biomater. 2010, 3, 119–139. [Google Scholar] [CrossRef]
- Al Saqr, A.; Khafagy, E.-S.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; Anwer, M.K.; Khan, S.; Lila, A.S.A.; Arab, H.H.; Hegazy, W.A. Synthesis of Aus by using green machinery: Characterization and in vitro toxicity. Nanomaterials 2021, 11, 808. [Google Scholar] [CrossRef]
- Qian, K.; Sweeny, B.C.; Johnston-Peck, A.C.; Niu, W.; Graham, J.O.; DuChene, J.S.; Qiu, J.; Wang, Y.-C.; Engelhard, M.H.; Su, D. Surface plasmon-driven water reduction: Ausize matters. J. Am. Chem. Soc. 2014, 136, 9842–9845. [Google Scholar] [CrossRef]
- Sarfraz, N.; Khan, I. Plasmonic Aus (AuNPs): Properties, synthesis and their advanced energy, environmental and biomedical applications. Chem.–Asian J. 2021, 16, 720–742. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The Potential Application of Green-Synthesized Metal Nanoparticles in Dentistry: A Comprehensive Review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef]
- Madkour, L.H. Nanoelectronic Materials: Fundamentals and Applications; Springer: New York, NY, USA, 2019; Volume 116. [Google Scholar]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Shen, C.-C.; Hsu, S.-h.; Chang, K.-B.; Yeh, C.-A.; Chang, H.-C.; Tang, C.-M.; Yang, Y.-C.; Hsieh, H.-H.; Hung, H.-S. Physical Au-Decorated Polyethylene Glycol-Hydroxyapatite Composites Guide Osteogenesis and Angiogenesis of Mesenchymal Stem Cells. Biomedicines 2021, 9, 1632. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Q. Protein-Auinteractions and their possible impact on biomedical applications. Acta Biomater. 2017, 55, 13–27. [Google Scholar] [CrossRef]
- Yu, A.Y.-H.; Fu, R.-H.; Hsu, S.-h.; Chiu, C.-F.; Fang, W.-H.; Yeh, C.-A.; Tang, C.-M.; Hsieh, H.-H.; Hung, H.-S. Epidermal growth factor receptors siRNA-conjugated collagen modified Aus for targeted imaging and therapy of lung cancer. Mater. Today Adv. 2021, 12, 100191. [Google Scholar] [CrossRef]
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical applications of functionalized Aus: A review. J. Clust. Sci. 2021, 33, 1–16. [Google Scholar] [CrossRef]
- Hung, H.-S.; Kung, M.-L.; Chen, F.-C.; Ke, Y.-C.; Shen, C.-C.; Yang, Y.-C.; Tang, C.-M.; Yeh, C.-A.; Hsieh, H.-H.; Hsu, S.-h. Nanogold-carried graphene oxide: Anti-inflammation and increased differentiation capacity of mesenchymal stem cells. Nanomaterials 2021, 11, 2046. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-S.; Yang, Y.-C.; Kao, W.-C.; Yeh, C.-A.; Chang, K.-B.; Tang, C.-M.; Hsieh, H.-H.; Lee, H.-T. Evaluation of the Biocompatibility and Endothelial Differentiation Capacity of Mesenchymal Stem Cells by Polyethylene Glycol Nanogold Composites. Polymers 2021, 13, 4265. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Del Bakhshayesh, A.R.; Davaran, S.; Akbarzadeh, A. Common biocompatible polymeric materials for tissue engineering and regenerative medicine. Mater. Chem. Phys. 2020, 242, 122528. [Google Scholar] [CrossRef]
- Gu, L.; Shan, T.; Ma, Y.-x.; Tay, F.R.; Niu, L. Novel biomedical applications of crosslinked collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Liu, A.; Wang, W. Improved thermal-stability and mechanical properties of type I collagen by crosslinking with casein, keratin and soy protein isolate using transglutaminase. Int. J. Biol. Macromol. 2017, 98, 292–301. [Google Scholar] [CrossRef]
- Xu, J.; Shi, G.-P. Vascular wall extracellular matrix proteins and vascular diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 2106–2119. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, S.-C.; Chen, H.-J.; Hsu, S.-H.; Yang, Y.-C.; Tang, C.-M.; Chu, M.-Y.; Lin, P.-Y.; Fu, R.-H.; Kung, M.-L.; Chen, Y.-W. Prominent vascularization capacity of mesenchymal stem cells in collagen–gold nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 28982–29000. [Google Scholar] [CrossRef]
- Chiu, C.-F.; Fu, R.-H.; Hsu, S.-h.; Yu, Y.-H.; Yang, S.-F.; Tsao, T.C.-Y.; Chang, K.-B.; Yeh, C.-A.; Tang, C.-M.; Huang, S.-C. Delivery Capacity and Anticancer Ability of the Berberine-Loaded Aus to Promote the Apoptosis Effect in Breast Cancer. Cancers 2021, 13, 5317. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-Y.; Lin, C.-H.; Ho, T.-T.; Chen, H.-C.; Chu, M.-Y.; Sun, W.-S.; Kao, W.-C.; Hung, H.-S.; Hsu, S.-h. Enhanced migration of wharton’s jelly mesenchymal stem cells grown on polyurethane nanocomposites. J. Med. Biol. Eng. 2013, 33, 139–148. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Hsieh, S.-C.; Yang, Y.-C.; Hsu, S.-h.; Kung, M.-L.; Lin, P.-Y.; Hsieh, H.-H.; Lin, C.-H.; Tang, C.-M.; Hung, H.-S. Functional engineered mesenchymal stem cells with fibronectin-gold composite coated catheters for vascular tissue regeneration. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 699–711. [Google Scholar] [CrossRef]
- Yi, C.; Liu, D.; Fong, C.-C.; Zhang, J.; Yang, M. Aus promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Cao, H.-B.; Li, W.-J.; Li, Z. The CXCL12 (SDF-1)/CXCR4 chemokine axis: Oncogenic properties, molecular targeting, and synthetic and natural product CXCR4 inhibitors for cancer therapy. Chin. J. Nat. Med. 2018, 16, 801–810. [Google Scholar]
- Adamiak, M.; Borkowska, S.; Wysoczynski, M.; Suszynska, M.; Kucia, M.; Rokosh, G.; Abdel-Latif, A.; Ratajczak, J.; Ratajczak, M.Z. Evidence for the involvement of sphingosine-1-phosphate in the homing and engraftment of hematopoietic stem cells to bone marrow. Oncotarget 2015, 6, 18819–18828. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Yao, X.; Wu, W.; Yu, Z.; Li, Z.; Ma, Z.; Liu, D. HIF-1α/SDF-1/CXCR4 axis reduces neuronal apoptosis via enhancing the bone marrow-derived mesenchymal stromal cell migration in rats with traumatic brain injury. Exp. Mol. Pathol. 2020, 114, 104416. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, A.; Tao, C.; Li, X.; Jin, P. The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochem. Biophys. Res. Commun. 2013, 441, 675–680. [Google Scholar] [CrossRef]
- Hung, H.-S.; Kao, W.-C.; Shen, C.-C.; Chang, K.-B.; Tang, C.-M.; Yang, M.-Y.; Yang, Y.-C.; Yeh, C.-A.; Li, J.-J.; Hsieh, H.-H. Inflammatory Modulation of Polyethylene Glyc.ol-AuNP for Regulation of the Neural Differentiation Capacity of Mesenchymal Stem Cells. Cells 2021, 10, 2854. [Google Scholar] [CrossRef]
- Xiao, H.; Cai, G.; Liu, M. Hydroxyl radical induced structural changes of collagen. Spectroscopy 2007, 21, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dar, A.; Kollet, O.; Lapidot, T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp. Hematol. 2006, 34, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Jones, D.; Borghesani, P.R.; Segal, R.A.; Nagasawa, T.; Kishimoto, T.; Bronson, R.T.; Springer, T.A. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4-and SDF-1-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 9448–9453. [Google Scholar] [CrossRef]
- Malgieri, A.; Kantzari, E.; Patrizi, M.P.; Gambardella, S. Bone marrow and umbilical cord blood human mesenchymal stem cells: State of the art. Int. J. Clin. Exp. Med. 2010, 3, 248. [Google Scholar]
- da Silva Meirelles, L.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef]
- Li, H.; Pan, S.; Xia, P.; Chang, Y.; Fu, C.; Kong, W.; Yu, Z.; Wang, K.; Yang, X.; Qi, Z. Advances in the application of Aus in bone tissue engineering. J. Biol. Eng. 2020, 14, 14. [Google Scholar] [CrossRef]
- Gupta, V.K.; Chaudhuri, O. Mechanical regulation of cell-cycle progression and division. Trends Cell Biol. 2022, 32, 773–785. [Google Scholar] [CrossRef]
- Kupcho, K.; Shultz, J.; Hurst, R.; Hartnett, J.; Zhou, W.; Machleidt, T.; Grailer, J.; Worzella, T.; Riss, T.; Lazar, D. A real-time, bioluminescent annexin V assay for the assessment of apoptosis. Apoptosis 2019, 24, 184–197. [Google Scholar] [CrossRef] [Green Version]
- Baldin, V.; Lukas, J.; Marcote, M.J.; Pagano, M.; Draetta, G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993, 7, 812–821. [Google Scholar] [CrossRef] [Green Version]
- Gartel, A.L.; Serfas, M.S.; Tyner, A.L. p21—Negative regulator of the cell cycle. Proc. Soc. Exp. Biol. Med. 1996, 213, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Pisani, C.; Ramella, M.; Boldorini, R.; Loi, G.; Billia, M.; Boccafoschi, F.; Volpe, A.; Krengli, M. Apoptotic and predictive factors by Bax, Caspases 3/9, Bcl-2, p53 and Ki-67 in prostate cancer after 12 Gy single-dose. Sci. Rep. 2020, 10, 7050. [Google Scholar] [CrossRef]
- D’Aguanno, S.; Del Bufalo, D. Inhibition of anti-apoptotic Bcl-2 proteins in preclinical and clinical studies: Current overview in cancer. Cells 2020, 9, 1287. [Google Scholar] [CrossRef] [PubMed]
- Bernal, A.; Arranz, L. Nestin-expressing progenitor cells: Function, identity and therapeutic implications. Cell. Mol. Life Sci. 2018, 75, 2177–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, F.; Paulin, D.; Moura Neto, V. Glial fibrillary acidic protein (GFAP): Modulation by growth factors and its implication in astrocyte differentiation. Braz. J. Med. Biol. Res. 1999, 32, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Downing, K.H.; Nogales, E. Tubulin and microtubule structure. Curr. Opin. Cell Biol. 1998, 10, 16–22. [Google Scholar] [CrossRef]
- Chen, H.-C.; Kung, M.-l.; Huang, W.-C.; Fu, R.-H.; Yang, H.-Y.; Yang, Y.-T.; Hung, H.-S. Delivery of stromal-derived factor-1α via biocompatible gold nanoparticles promotes dendritic cells viability and migration. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127298. [Google Scholar] [CrossRef]
- Falcone, S.; Cocucci, E.; Podini, P.; Kirchhausen, T.; Clementi, E.; Meldolesi, J. Macropinocytosis: Regulated coordination of endocytic and exocytic membrane traffic events. J. Cell Sci. 2006, 119, 4758–4769. [Google Scholar] [CrossRef] [Green Version]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [Green Version]
- Daniel, J.A.; Chau, N.; Abdel-Hamid, M.K.; Hu, L.; von Kleist, L.; Whiting, A.; Krishnan, S.; Maamary, P.; Joseph, S.R.; Simpson, F. Phenothiazine-derived antipsychotic drugs inhibit dynamin and clathrin-mediated endocytosis. Traffic 2015, 16, 635–654. [Google Scholar] [CrossRef]
- Llanos, P.; Contreras-Ferrat, A.; Georgiev, T.; Osorio-Fuentealba, C.; Espinosa, A.; Hidalgo, J.; Hidalgo, C.; Jaimovich, E. The cholesterol-lowering agent methyl-β-cyclodextrin promotes glucose uptake via GLUT4 in adult muscle fibers and reduces insulin resistance in obese mice. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E294–E305. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Sintiprungrat, K.; Chaiyarit, S.; Thongboonkerd, V. Macropinocytosis is the major mechanism for endocytosis of calcium oxalate crystals into renal tubular cells. Cell Biochem. Biophys. 2013, 67, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Manabe, T.; Yoshimori, T.; Henomatsu, N.; Tashiro, Y. Inhibitors of vacuolar-type H+-ATPase suppresses proliferation of cultured cells. J. Cell. Physiol. 1993, 157, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Shirihai, O.S.; Grinstaff, M.W. Modulating lysosomal pH: A molecular and nanoscale materials design perspective. J. Life Sci. 2020, 2, 25. [Google Scholar] [CrossRef] [PubMed]
















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.-Y.; Chiu, C.-D.; Ke, Y.-C.; Yang, Y.-C.; Chang, K.-B.; Chen, C.-M.; Lee, H.-T.; Tang, C.-L.; Liu, B.-S.; Hung, H.-S. Differentiation Induction of Mesenchymal Stem Cells by a Au Delivery Platform. Cells 2023, 12, 1893. https://doi.org/10.3390/cells12141893
Yang M-Y, Chiu C-D, Ke Y-C, Yang Y-C, Chang K-B, Chen C-M, Lee H-T, Tang C-L, Liu B-S, Hung H-S. Differentiation Induction of Mesenchymal Stem Cells by a Au Delivery Platform. Cells. 2023; 12(14):1893. https://doi.org/10.3390/cells12141893
Chicago/Turabian StyleYang, Meng-Yin, Cheng-Di Chiu, Yi-Chun Ke, Yi-Chin Yang, Kai-Bo Chang, Chien-Min Chen, Hsu-Tung Lee, Chien-Lun Tang, Bai-Shuan Liu, and Huey-Shan Hung. 2023. "Differentiation Induction of Mesenchymal Stem Cells by a Au Delivery Platform" Cells 12, no. 14: 1893. https://doi.org/10.3390/cells12141893
APA StyleYang, M. -Y., Chiu, C. -D., Ke, Y. -C., Yang, Y. -C., Chang, K. -B., Chen, C. -M., Lee, H. -T., Tang, C. -L., Liu, B. -S., & Hung, H. -S. (2023). Differentiation Induction of Mesenchymal Stem Cells by a Au Delivery Platform. Cells, 12(14), 1893. https://doi.org/10.3390/cells12141893