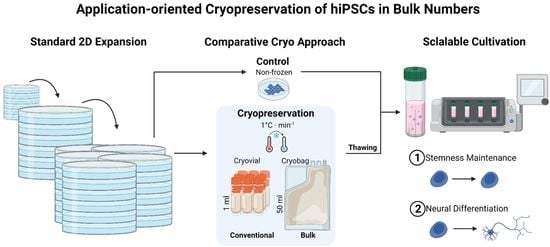
Application-Oriented Bulk Cryopreservation of Human iPSCs in Cryo Bags Followed by Direct Inoculation in Scalable Suspension Bioreactors for Expansion and Neural Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. hiPSC Culture
2.2. Neural Differentiation
2.3. Cryopreservation Procedures
2.4. Thawing Procedures
2.5. Cell Counting, Viability, and Aggregation Rate Assessment
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Immunocytochemical Staining (ICC)
2.8. Flow Cytometry (FCM)
2.9. Statistical Analysis
3. Results
3.1. Direct Inoculation of a Suspension Bioreactor with Cells Frozen in a Bulk Cryopreservation Approach
3.2. Performance of Bulk-Cryopreserved Cells in Bags Regarding Cultivation Parameters
3.3. Performance of Bulk-Cryopreserved Cells in Bags Regarding Maintenanceof Pluripotency Characteristics
3.4. Performance of Bulk-Cryopreserved Cells in Bags, Regarding Their Differentiation Capacity
4. Discussion
4.1. Pre-Studies Determined Cell Concentration and ROCK Inhibition
4.2. More Homogeneous Aggregates after Bulk Freezing in Bags
4.3. Maintenance of Stemness upon Thawing and Bioreactor Cultivation
4.4. Cell-Line Specific Differences in Performance
4.5. Neuronal Differentiation Capacity Maintained but Delayed
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Name | Cat. Nr. | Source |
|---|---|---|
| 2-Mercaptoethanol | 31350010 | Gibco™ |
| B-27 Supplement | 17504044 | Gibco™ |
| BD Cytofix™ Fixation Buffer | 554655 | BD Biosciences |
| BD Perm/Wash Buffer | 554723 | BD Biosciences |
| Bovine serum albumin | 0332-25G | VWR |
| CryoStor® CS10 | 07930 | Stemcell™ Technologies |
| DMEM/F-12 (+ L-glutamine/+ 15 mM HEPES) | 11330057 | Gibco™ |
| DMEM/F-12, GlutaMAX™ supplement | 31331028 | Gibco™ |
| Doxycycline | 04-0016 | Stemgent |
| EDTA | AM9260G | Invitrogen™ |
| Embryoid Body Dissociation Kit | 130-096-348 | Miltenyi |
| Fetal bovine serum (FBS) | 10500-064 | Gibco™ |
| GlutaMAX™ Supplement | 35050038 | Gibco™ |
| High-Capacity cDNA Reverse Transcription Kit | 4368814 | Applied Biosystems™ |
| human Insulin solution | I9278 | Sigma-Aldrich |
| Lysis buffer A100 | 910-0010 | Chemometec |
| Matrigel® | 354230 | Corning® |
| MEM Non-EAAS | 11140035 | Gibco™ |
| mTeSR™1 medium | 85850 | Stemcell™ Technologies |
| N-2 Supplement | 17502048 | Gibco™ |
| Neurobasal Medium | 21103049 | Gibco™ |
| Penicillin-Streptomycin-Glutamin | 10378016 | Gibco™ |
| Phosphate buffered saline | 11540546 | Gibco™ |
| RNeasy Micro Kit | 74004 | Qiagen |
| Sodium azide (NaN3) | S2002 | Sigma Aldrich |
| Sodium Pyruvate | 11360070 | Gibco™ |
| Stabilisation buffer B | 910-0002 | Chemometec |
| TaqMan® Fast Advanced Master Mix | 4444557 | Applied Biosystems™ |
| Triton™ X-100 | T878-100ML | Sigma-Aldrich |
| TrypLE™ Select | 11598846 | Gibco™ |
| Tween® 80 | P4780-100ML | Sigma-Aldrich |
| Y-27632 | 10005583 | Cayman Chemicals |
| Target | TaqMan Assay ID | Source |
|---|---|---|
| GAPDH | Hs99999905_m1 | Life Tech, Thermo Fisher Scientific |
| HPRT1 | Hs99999909_m1 | |
| POU5F1 | Hs00742896_s1 | |
| NANOG | Hs04399610_g1 | |
| SOX2 | Hs00602736_s1 | |
| PAX6 | Hs01088114_m1 | |
| GATA6 | Hs00232018_m1 | |
| VIM | Hs00185584_m1 | |
| HES5 | Hs01387463_g1 | |
| NESTIN | Hs04187831_g1 | |
| NEUROD1 | Hs01922995_s1 | |
| TUBB3 | Hs00964962_g1 | |
| MAP2 | Hs00258900_m1 | |
| FOXG1 | Hs01850784_s1 | |
| MAPT | Hs00902194_m1 |
| Antibody | Host Species | Isotype | Dilution | Cat. Nr. | Source |
|---|---|---|---|---|---|
| TRA-1-60-R | Mouse | IgM, κ | 1:400 | 330602 | BioLegend® |
| Tubulin β 3 (Clone: TUJ1) | Mouse | IgG2a, κ | 1:400 | 801201 | BioLegend® |
| MAP2 | Rabbit | IgG | 1:100 | 840601 | BioLegend® |
| Antibody | Host Species | Isotype | Fluorophor | Dilution | Cat. Nr. | Source |
|---|---|---|---|---|---|---|
| Anti-Mouse | Donkey | IgG H + L | Alexa FluorTM 488 | 1:10,000 | A21202 | Thermo Fisher Scientific |
| Anti-Rabbit | Goat | IgG | Alexa Fluor™ 594 | 2 drops/mL | R37117 | Invitrogen |
| Antibody | Host Species | Isotype | Fluorophor | Dilution | Cat. Nr. | Source |
|---|---|---|---|---|---|---|
| Oct3/4 | Mouse | IgG1, κ | PerCP-CyTM5.5 | 1:5 | 560794 | BD Biosciences |
| TRA-1-60-R | Mouse | IgM, κ | Alexa Fluor® 647 | 1:20 (5µL in 100 µL staining volume) | 330606 | BioLegend® |
| CD15 SSEA-1 | Mouse | IgM, κ | PE/Cyanine7 | 1:20 | 301924 | BioLegend® |
| Isotype Ctrl | Mouse | IgM, κ | PE/Cyanine7 | 1:20 | 401628 | BioLegend® |
| SSEA4 | Human | - | FITC | 1:5 | 130-098-371 | Miltenyi Biotec |
| anti-β-Tubulin, Class III | Mouse | IgM, κ | Alexa Fluor® 647 | 1:20 | 558606 | BD Biosciences |
References
- Nishikawa, S.; Goldstein, R.A.; Nierras, C.R. The promise of human induced pluripotent stem cells for research and therapy. Nat. Rev. Mol. Cell Biol. 2008, 9, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, E.I.; Scalise, S.; Scaramuzzino, L.; Cuda, G. Stem Cells: The Game Changers of Human Cardiac Disease Modelling and Regenerative Medicine. Int. J. Mol. Sci. 2019, 20, 5760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induced pluripotent stem cells in medicine and biology. Development 2013, 140, 2457–2461. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- Steeg, R.; Neubauer, J.C.; Müller, S.C.; Ebneth, A.; Zimmermann, H. The EBiSC iPSC bank for disease studies. Stem Cell Res. 2020, 49, 102034. [Google Scholar] [CrossRef]
- de Sousa, P.A.; Steeg, R.; Wachter, E.; Bruce, K.; King, J.; Hoeve, M.; Khadun, S.; McConnachie, G.; Holder, J.; Kurtz, A.; et al. Rapid establishment of the European Bank for induced Pluripotent Stem Cells (EBiSC)—The Hot Start experience. Stem Cell Res. 2017, 20, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Stacey, G. Stem Cell Banking: A Global View. Methods Mol. Biol. 2017, 1590, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kato, T.M.; Sato, Y.; Umekage, M.; Ichisaka, T.; Tsukahara, M.; Takasu, N.; Yamanaka, S. A clinical-grade HLA haplobank of human induced pluripotent stem cells matching approximately 40% of the Japanese population. Med 2023, 4, 51–66.e10. [Google Scholar] [CrossRef]
- Wrigley, J.D.; McCall, E.J.; Bannaghan, C.L.; Liggins, L.; Kendrick, C.; Griffen, A.; Hicks, R.; Fröderberg-Roth, L. Cell banking for pharmaceutical research. Drug Discov. Today 2014, 19, 1518–1529. [Google Scholar] [CrossRef]
- Sharma, S.; Raju, R.; Sui, S.; Hu, W.-S. Stem cell culture engineering—Process scale up and beyond. Biotechnol. J. 2011, 6, 1317–1329. [Google Scholar] [CrossRef]
- Serra, M.; Brito, C.; Correia, C.; Alves, P.M. Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol. 2012, 30, 350–359. [Google Scholar] [CrossRef]
- Vymetalova, L.; Kucirkova, T.; Knopfova, L.; Pospisilova, V.; Kasko, T.; Lejdarova, H.; Makaturova, E.; Kuglik, P.; Oralova, V.; Matalova, E.; et al. Large-Scale Automated Hollow-Fiber Bioreactor Expansion of Umbilical Cord-Derived Human Mesenchymal Stromal Cells for Neurological Disorders. Neurochem. Res. 2020, 45, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Borys, B.S.; Dang, T.; So, T.; Rohani, L.; Revay, T.; Walsh, T.; Thompson, M.; Argiropoulos, B.; Rancourt, D.E.; Jung, S.; et al. Overcoming bioprocess bottlenecks in the large-scale expansion of high-quality hiPSC aggregates in vertical-wheel stirred suspension bioreactors. Stem Cell Res. Ther. 2021, 12, 55. [Google Scholar] [CrossRef]
- Davis, B.M.; Loghin, E.R.; Conway, K.R.; Zhang, X. Automated Closed-System Expansion of Pluripotent Stem Cell Aggregates in a Rocking-Motion Bioreactor. SLAS Technol. 2018, 23, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Elanzew, A.; Nießing, B.; Langendoerfer, D.; Rippel, O.; Piotrowski, T.; Schenk, F.; Kulik, M.; Peitz, M.; Breitkreuz, Y.; Jung, S.; et al. The StemCellFactory: A Modular System Integration for Automated Generation and Expansion of Human Induced Pluripotent Stem Cells. Front. Bioeng. Biotechnol. 2020, 8, 580352. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.K.; Sébastien, I.; Hariharan, K.; Meiser, I.; Wihan, J.; Altmaier, S.; Karnatz, I.; Bauer, D.; Fischer, B.; Feile, A.; et al. Scalable expansion of iPSC and their derivatives across multiple lineages. Reprod. Toxicol. 2022, 112, 23–35. [Google Scholar] [CrossRef]
- Altmaier, S.; Meiser, I.; Lemesre, E.; Chanrion, B.; Steeg, R.; Leonte, L.E.; Holst, B.; Nielsen, B.S.; Clausen, C.; Schmidt, K.; et al. Human iPSC-derived hepatocytes in 2D and 3D suspension culture for cryopreservation and in vitro toxicity studies. Reprod. Toxicol. 2022, 111, 68–80. [Google Scholar] [CrossRef]
- Badenes, S.M.; Fernandes, T.G.; Rodrigues, C.A.V.; Diogo, M.M.; Cabral, J.M.S. Microcarrier-based platforms for in vitro expansion and differentiation of human pluripotent stem cells in bioreactor culture systems. J. Biotechnol. 2016, 234, 71–82. [Google Scholar] [CrossRef]
- Azarin, S.M.; Palecek, S.P. Matrix revolutions: A trinity of defined substrates for long-term expansion of human ESCs. Cell Stem Cell 2010, 7, 7–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerke, S.; Taupitz, J.; Wiesemann, C.; Kopetzki, C.; Zimmermann, H. Die klinische Anwendung von humanen induzierten pluripotenten Stammzellen; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-662-59051-5. [Google Scholar]
- Meneghel, J.; Kilbride, P.; Morris, G.J. Cryopreservation as a Key Element in the Successful Delivery of Cell-Based Therapies—A Review. Front. Med. 2020, 7, 592242. [Google Scholar] [CrossRef]
- Shibamiya, A.; Schulze, E.; Krauß, D.; Augustin, C.; Reinsch, M.; Schulze, M.L.; Steuck, S.; Mearini, G.; Mannhardt, I.; Schulze, T.; et al. Cell Banking of hiPSCs: A Practical Guide to Cryopreservation and Quality Control in Basic Research. Curr. Protoc. Stem Cell Biol. 2020, 55, e127. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Hirsh, A.; Erbe, E.; Williams, R.J. Mechanism of cryoprotection by extracellular polymeric solutes. Biophys. J. 1988, 54, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazur, P. Freezing of living cells: Mechanisms and implications. Am. J. Physiol. 1984, 247, C125–C142. [Google Scholar] [CrossRef] [PubMed]
- Steeg, R.; Mueller, S.C.; Mah, N.; Holst, B.; Cabrera-Socorro, A.; Stacey, G.N.; de Sousa, P.A.; Courtney, A.; Zimmermann, H. EBiSC best practice: How to ensure optimal generation, qualification, and distribution of iPSC lines. Stem Cell Rep. 2021, 16, 1853–1867. [Google Scholar] [CrossRef]
- Heidemann, R.; Lünse, S.; Tran, D.; Zhang, C. Characterization of cell-banking parameters for the cryopreservation of mammalian cell lines in 100-mL cryobags. Biotechnol. Prog. 2010, 26, 1154–1163. [Google Scholar] [CrossRef]
- Spoerl, S.; Peter, R.; Krackhardt, A.M. Cryopreservation in Closed Bag Systems as an Alternative to Clean Rooms for Preparations of Peripheral Blood Stem Cells. Biobanking and Cryopreservation of Stem Cells; Springer: Cham, Switzerland, 2016; pp. 67–76. [Google Scholar]
- Das, A.T.; Tenenbaum, L.; Berkhout, B. Tet-On Systems For Doxycycline-inducible Gene Expression. Curr. Gene Ther. 2016, 16, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.-M.; Hartley, B.J.; Tcw, J.; Beaumont, M.; Stafford, K.; Slesinger, P.A.; Brennand, K.J. Rapid Ngn2-induction of excitatory neurons from hiPSC-derived neural progenitor cells. Methods 2016, 101, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; van Hoang, T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Bashor, C.J.; Hilton, I.B.; Bandukwala, H.; Smith, D.M.; Veiseh, O. Engineering the next generation of cell-based therapeutics. Nat. Rev. Drug Discov. 2022, 21, 655–675. [Google Scholar] [CrossRef]
- O’Shea, O.; Steeg, R.; Chapman, C.; Mackintosh, P.; Stacey, G.N. Development and implementation of large-scale quality control for the European bank for induced Pluripotent Stem Cells. Stem Cell Res. 2020, 45, 101773. [Google Scholar] [CrossRef]
- Andrews, P.W.; Baker, D.; Benvinisty, N.; Miranda, B.; Bruce, K.; Brüstle, O.; Choi, M.; Choi, Y.-M.; Crook, J.M.; de Sousa, P.A.; et al. Points to consider in the development of seed stocks of pluripotent stem cells for clinical applications: International Stem Cell Banking Initiative (ISCBI). Regen. Med. 2015, 10, 1–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manstein, F.; Halloin, C.; Zweigerdt, R. Human Pluripotent Stem Cell Expansion in Stirred Tank Bioreactors. Methods Mol. Biol. 2019, 1994, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.J.R.; Luquet, E.; Pletenka, J.; Leonard, A.; Warter, E.; Gurchenkov, B.; Carrere, J.; Rieu, C.; Hardouin, J.; Moncaubeig, F.; et al. Engineering 3D micro-compartments for highly efficient and scale-independent expansion of human pluripotent stem cells in bioreactors. Biomaterials 2023, 295, 122033. [Google Scholar] [CrossRef]
- Massie, I.; Selden, C.; Hodgson, H.; Fuller, B.; Gibbons, S.; Morris, G.J. GMP cryopreservation of large volumes of cells for regenerative medicine: Active control of the freezing process. Tissue Eng. Part C Methods 2014, 20, 693–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Mai, Q.; Gao, J.; Zhou, C. Cryopreservation of human embryonic stem cells with a new bulk vitrification method. Biol. Reprod. 2010, 82, 848–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Ma, T. Bioprocessing of cryopreservation for large-scale banking of human pluripotent stem cells. Biores. Open Access 2012, 1, 205–214. [Google Scholar] [CrossRef]
- Hunt, C.J. Technical Considerations in the Freezing, Low-Temperature Storage and Thawing of Stem Cells for Cellular Therapies. Transfus. Med. Hemother. 2019, 46, 134–150. [Google Scholar] [CrossRef]
- Murray, K.A.; Gibson, M.I. Chemical approaches to cryopreservation. Nat. Rev. Chem. 2022, 6, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.; Gonzalez-Molina, J.; Erro, E.; de Mendonca, J.; Chalmers, S.; Awan, M.; Poirier, A.; Selden, C. Applications and optimization of cryopreservation technologies to cellular therapeutics. Cell Gene Ther. Insights 2017, 3, 359–378. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Muguruma, K.; Sasai, Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef]
- Li, X.; Meng, G.; Krawetz, R.; Liu, S.; Rancourt, D.E. The ROCK inhibitor Y-27632 enhances the survival rate of human embryonic stem cells following cryopreservation. Stem Cells Dev. 2008, 17, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Nakatsuji, N.; Suemori, H. Optimization of slow cooling cryopreservation for human pluripotent stem cells. Genesis 2014, 52, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.; Yoshie, S.; Shirasawa, S.; Yokoyama, T.; Yue, F.; Tomotsune, D.; Sasaki, K. Freeze-thawing single human embryonic stem cells induce e-cadherin and actin filament network disruption via g13 signaling. Cryo Lett. 2011, 32, 516–524. [Google Scholar]
- Vernardis, S.I.; Terzoudis, K.; Panoskaltsis, N.; Mantalaris, A. Human embryonic and induced pluripotent stem cells maintain phenotype but alter their metabolism after exposure to ROCK inhibitor. Sci. Rep. 2017, 7, 42138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, C.J. Cryopreservation of Human Stem Cells for Clinical Application: A Review. Transfus. Med. Hemotherapy Off. Organ Dtsch. Ges. Transfusionsmedizin Immunhamatol. 2011, 38, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Yirme, G.; Amit, M.; Laevsky, I.; Osenberg, S.; Itskovitz-Eldor, J. Establishing a dynamic process for the formation, propagation, and differentiation of human embryoid bodies. Stem Cells Dev. 2008, 17, 1227–1241. [Google Scholar] [CrossRef]
- Baboo, J.; Kilbride, P.; Delahaye, M.; Milne, S.; Fonseca, F.; Blanco, M.; Meneghel, J.; Nancekievill, A.; Gaddum, N.; Morris, G.J. The Impact of Varying Cooling and Thawing Rates on the Quality of Cryopreserved Human Peripheral Blood T Cells. Sci. Rep. 2019, 9, 3417. [Google Scholar] [CrossRef] [Green Version]
- Mazur, P.; Leibo, S.P.; Chu, E.H. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp. Cell Res. 1972, 71, 345–355. [Google Scholar] [CrossRef]
- van Hoof, D.; Braam, S.R.; Dormeyer, W.; Ward-van Oostwaard, D.; Heck, A.J.R.; Krijgsveld, J.; Mummery, C.L. Feeder-free monolayer cultures of human embryonic stem cells express an epithelial plasma membrane protein profile. Stem Cells 2008, 26, 2777–2781. [Google Scholar] [CrossRef]
- Rouhani, F.; Kumasaka, N.; de Brito, M.C.; Bradley, A.; Vallier, L.; Gaffney, D. Genetic background drives transcriptional variation in human induced pluripotent stem cells. PLoS Genet. 2014, 10, e1004432. [Google Scholar] [CrossRef]
- Katkov, I.I.; Kim, M.S.; Bajpai, R.; Altman, Y.S.; Mercola, M.; Loring, J.F.; Terskikh, A.V.; Snyder, E.Y.; Levine, F. Cryopreservation by slow cooling with DMSO diminished production of Oct-4 pluripotency marker in human embryonic stem cells. Cryobiology 2006, 53, 194–205. [Google Scholar] [CrossRef]
- Xu, X.; Cowley, S.; Flaim, C.J.; James, W.; Seymour, L.; Cui, Z. The roles of apoptotic pathways in the low recovery rate after cryopreservation of dissociated human embryonic stem cells. Biotechnol. Prog. 2010, 26, 827–837. [Google Scholar] [CrossRef]
- Len, J.S.; Koh, W.S.D.; Tan, S.-X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, M.; Grealish, S.; Henchcliffe, C. The future of stem cell therapies for Parkinson disease. Nat. Rev. Neurosci. 2020, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Jang, J. Directed Differentiation of Pluripotent Stem Cells by Transcription Factors. Mol. Cells 2019, 42, 200–209. [Google Scholar] [CrossRef]
- Shao, Q.; Yang, T.; Huang, H.; Alarmanazi, F.; Liu, G. Uncoupling of UNC5C with Polymerized TUBB3 in Microtubules Mediates Netrin-1 Repulsion. J. Neurosci. 2017, 37, 5620–5633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanov-Milošević, N.; Petanjek, Z.; Petrović, D.; Judaš, M.; Kostović, I. Morphology, molecular phenotypes and distribution of neurons in developing human corpus callosum. Eur. J. Neurosci. 2010, 32, 1423–1432. [Google Scholar] [CrossRef]
- Ortiz-Rodriguez, J.M.; Ortega-Ferrusola, C.; Gil, M.C.; Martín-Cano, F.E.; Gaitskell-Phillips, G.; Rodríguez-Martínez, H.; Hinrichs, K.; Álvarez-Barrientos, A.; Román, Á.; Peña, F.J. Transcriptome analysis reveals that fertilization with cryopreserved sperm downregulates genes relevant for early embryo development in the horse. PLoS ONE 2019, 14, e0213420. [Google Scholar] [CrossRef] [Green Version]
- Paasch, U.; Sharma, R.K.; Gupta, A.K.; Grunewald, S.; Mascha, E.J.; Thomas, A.J.; Glander, H.-J.; Agarwal, A. Cryopreservation and thawing is associated with varying extent of activation of apoptotic machinery in subsets of ejaculated human spermatozoa. Biol. Reprod. 2004, 71, 1828–1837. [Google Scholar] [CrossRef] [Green Version]
- Pegg, D.E. Principles of cryopreservation. Methods Mol. Biol. 2015, 1257, 3–19. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meiser, I.; Alstrup, M.; Khalesi, E.; Stephan, B.; Speicher, A.M.; Majer, J.; Kwok, C.K.; Neubauer, J.C.; Hansson, M.; Zimmermann, H. Application-Oriented Bulk Cryopreservation of Human iPSCs in Cryo Bags Followed by Direct Inoculation in Scalable Suspension Bioreactors for Expansion and Neural Differentiation. Cells 2023, 12, 1914. https://doi.org/10.3390/cells12141914
Meiser I, Alstrup M, Khalesi E, Stephan B, Speicher AM, Majer J, Kwok CK, Neubauer JC, Hansson M, Zimmermann H. Application-Oriented Bulk Cryopreservation of Human iPSCs in Cryo Bags Followed by Direct Inoculation in Scalable Suspension Bioreactors for Expansion and Neural Differentiation. Cells. 2023; 12(14):1914. https://doi.org/10.3390/cells12141914
Chicago/Turabian StyleMeiser, Ina, Monica Alstrup, Elham Khalesi, Bianca Stephan, Anna M. Speicher, Julia Majer, Chee Keong Kwok, Julia C. Neubauer, Mattias Hansson, and Heiko Zimmermann. 2023. "Application-Oriented Bulk Cryopreservation of Human iPSCs in Cryo Bags Followed by Direct Inoculation in Scalable Suspension Bioreactors for Expansion and Neural Differentiation" Cells 12, no. 14: 1914. https://doi.org/10.3390/cells12141914
APA StyleMeiser, I., Alstrup, M., Khalesi, E., Stephan, B., Speicher, A. M., Majer, J., Kwok, C. K., Neubauer, J. C., Hansson, M., & Zimmermann, H. (2023). Application-Oriented Bulk Cryopreservation of Human iPSCs in Cryo Bags Followed by Direct Inoculation in Scalable Suspension Bioreactors for Expansion and Neural Differentiation. Cells, 12(14), 1914. https://doi.org/10.3390/cells12141914