The Emerging Role of the Microbiota in Breast Cancer Progression
Abstract
:1. Introduction
2. Relationship between Breast Cancer and the Gut Microbiome
2.1. Gut Microbes–Immunity Crosstalk
2.2. Modulation of Estrogen Levels
2.3. Role of Microbial Metabolites
3. Breast Microbiome and Its Impact on Breast Cancer
| Taxa Enriched in TNBCs | Reference |
|---|---|
| Actinomycetaceae, Caulobacteriaceae, Sphingobacteriaceae, Enterobacteriaceae, prevotellaceae, Brucellaceae, Bacillaceae, Peptostreptococcaceae, Flavobacteriaceae | [62] |
| Actinomyces, Bartonella, Brevundimonas, Coxiella, Mobiluncus, Mycobacterium, Rickettsia, Sphingomonas | [66] |
| Azomonas, Alkanindiges, Caulobacter, Proteus, Brevibacillus, Kocuria, Parasediminibacterium | [67] |
3.1. Mechanistic Role of Breast Microbiome in the Progression of Breast Cancer
3.1.1. Carcinogenic Effect on the Host Genome
3.1.2. Effect on Cell Growth/Apoptosis
3.1.3. Effects on the Immunity
3.1.4. Microbial Metabolites Production
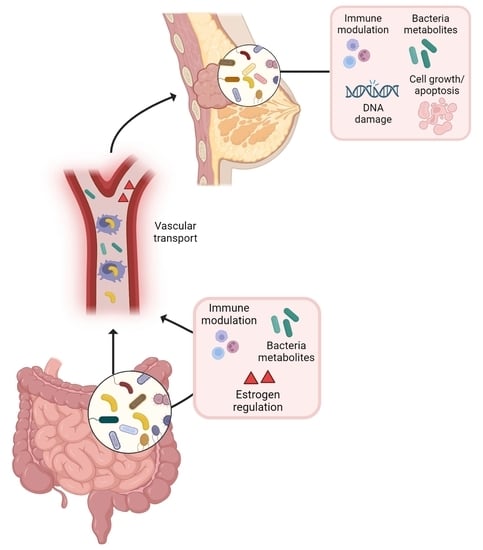
4. The Gut–Breast Microbiota Axis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. Available online: https://pubmed.ncbi.nlm.nih.gov/26824647/ (accessed on 28 January 2016). [CrossRef] [PubMed] [Green Version]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Battoet, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Kashtanova, D.A.; Tkacheva, O.N.; Doudinskaya, E.N.; Strazhesko, I.D.; Kotovskaya, Y.V.; Popenko, A.S.; Tyakht, A.V.; Alexeev, D.G. Gut Microbiota in Patients with Different Metabolic Statuses: Moscow Study. Microorganisms 2018, 6, 98. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6313665/ (accessed on 15 September 2018). [CrossRef] [Green Version]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science 2006, 312, 1355–1359. Available online: https://pubmed.ncbi.nlm.nih.gov/16741115/ (accessed on 2 June 2006). [CrossRef] [PubMed] [Green Version]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Sig. Transduct. Target. Ther. 2022, 7, 135. Available online: https://www.nature.com/articles/s41392-022-00974-4 (accessed on 23 April 2022).
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Baban, C.K.; Cronin, M.; O’hanlon, D.; O’sullivan, G.C.; Tangney, M. Bacteria as vectors for gene therapy of cancer. Bioeng. Bugs 2010, 1, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Franciset, K.P.; et al. Microbiota of human breast tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, H.; MacSharry, J.; Bueso, Y.F.; Lindsay, S.; Kavanagh, E.L.; Tangney, M.; Clyne, M.; Saldova, R.; McCann, A. Resident Bacteria in Breast Cancer Tissue: Pathogenic Agents or Harmless Commensals? Discov. Med. 2018, 26, 93–102. Available online: https://pubmed.ncbi.nlm.nih.gov/30399327/ (accessed on 28 September 2018).
- Hieken, T.J.; Chen, J.; Hoskin, T.L.; Walther-Antonio, M.; Johnson, S.; Ramaker, S.; Xiao, J.; Radisky, D.C.; Knutson, K.L.; Kalari, K.R.; et al. The Microbiome of Aseptically Collected Human Breast Tissue in Benign and Malignant Disease. Sci. Rep. 2016, 6, 30751. Available online: https://www.nature.com/articles/srep30751/ (accessed on 3 August 2016). [CrossRef] [PubMed]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Peck, K.N.; DeMichele, A.M.; Alwine, J.C.; Robertson, E.S. Distinct Microbial Signatures Associated With Different Breast Cancer Types. Front. Microbiol. 2018, 9, 951. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00951/full (accessed on 15 May 2018). [CrossRef] [PubMed] [Green Version]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. Available online: https://pubmed.ncbi.nlm.nih.gov/27342554/ (accessed on 29 July 2016). [CrossRef] [Green Version]
- Fu, A.; Yao, B.; Dong, T.; Chen, Y.; Yao, J.; Liu, Y.; Li, H.; Bai, H.; Liu, X.; Zhang, Y.; et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 2022, 185, 1356–1372.e26. [Google Scholar] [CrossRef]
- Bernardo, G.; Le Noci, V.; Ottaviano, E.; de Cecco, L.; Camisaschi, C.; Guglielmetti, S.; Di Modica, M.; Gargari, G.; Bianchi, F.; Indino, S.; et al. Reduction of Staphylococcus epidermidis in the mammary tumor microbiota induces antitumor immunity and decreases breast cancer aggressiveness. Cancer Lett. 2023, 555, 216041. [Google Scholar] [CrossRef]
- Ruo, S.W.; Alkayyali, T.; Win, M.; Tara, A.; Joseph, C.; Kannan, A.; Srivastava, K.; Ochuba, O.; Sandhu, J.K.; Went, T.R.; et al. Role of Gut Microbiota Dysbiosis in Breast Cancer and Novel Approaches in Prevention, Diagnosis, and Treatment. Cureus 2021, 13, e17472. [Google Scholar] [CrossRef]
- Alkabban, F.M.; Ferguson, T. Breast Cancer; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Breast Cancer: Subtypes of Breast Cancer; National Library of Medicine: Bethesda, MD, USA, 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/36122153/ (accessed on 6 August 2022).
- Dethlefsen, L.; McFall-Ngai, M.; Relman, D.A. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature 2007, 449, 811–818. [Google Scholar] [CrossRef]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the balance: Antibiotic effects on host–microbiota mutualism. Nat. Rev. Genet. 2011, 9, 233–243. [Google Scholar] [CrossRef]
- Arendt, L.M.; McCready, J.; Keller, P.J.; Baker, D.D.; Naber, S.P.; Seewaldt, V.; Kuperwasser, C. Obesity Promotes Breast Cancer by CCL2-Mediated Macrophage Recruitment and Angiogenesis. Cancer Res. 2013, 73, 6080–6093. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3824388/ (accessed on 1 October 2013). [CrossRef] [Green Version]
- Buchta Rosean, C.; Bostic, R.R.; Ferey, J.C.M.; Feng, T.-Y.; Azar, F.N.; Tung, K.S.; Dozmorov, M.G.; Smirnova, E.; Bos, P.D.; Rutkowski, P. Preexisting Commensal Dysbiosis Is a Host-Intrinsic Regulator of Tissue Inflammation and Tumor Cell Dissemination in Hormone Receptor-Positive Breast Cancer. Cancer Res. 2019, 79, 3662–3675. [Google Scholar] [CrossRef] [Green Version]
- Gwak, J.M.; Jang, M.H.; Kim, D.I.; Na Seo, A.; Park, S.Y. Prognostic Value of Tumor-Associated Macrophages According to Histologic Locations and Hormone Receptor Status in Breast Cancer. PLoS ONE 2015, 10, e0125728. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0125728 (accessed on 17 April 2015). [CrossRef] [PubMed]
- Buchta Rosean, C.; Feng, T.-Y.; Azar, F.N.; Rutkowski, M.R. Impact of the microbiome on cancer progression and response to anti-cancer therapies. Adv. Cancer Res. 2019, 143, 255–294. [Google Scholar] [PubMed]
- Lakritz, J.R.; Poutahidis, T.; Mirabal, S.; Varian, B.J.; Levkovich, T.; Ibrahim, Y.M.; Ward, J.M.; Teng, E.C.; Fisher, B.; Lesage, B.; et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget 2015, 6, 9387–9396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, K.C.; Araya, R.E.; Huang, A.; Chen, Q.; Di Modica, M.; Rodrigues, R.R.; Lopès, A.; Johnson, S.B.; Schwarz, B.; Bohrnsen, E.; et al. Microbiota Triggers STING-Type I IFN-Dependent Monocyte Reprogramming of the Tumor Microenvironment. Cell 2021, 184, 5338–5356.e21. Available online: https://www.sciencedirect.com/science/article/pii/S0092867421010667 (accessed on 7 October 2021). [CrossRef]
- Turning off Breast Cancer: A Personalized Approach to Nutrition and Detoxification in Prevention and Healing (Paperback)~Read. Available online: https://interesting-literature.gitlab.io/07-macie-yundt/turning-off-breast-cancer-a-personalized-approac-1632204452.pdf (accessed on 21 July 2015).
- Russo, J.; Russo, I.H. The Role of Estrogen in the Initiation of Breast Cancer. J. Steroid Biochem. Molecular Biol. 2006, 102, 89–96. Available online: https://pubmed.ncbi.nlm.nih.gov/17113977/ (accessed on 17 November 2006). [CrossRef] [Green Version]
- Zhu, B.T.; Conney, A.H. Functional role of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis 1998, 19, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Russo, J.; Moral, R.; A Balogh, G.; Mailo, D.; Russo, I.H. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005, 7, 131–142. [Google Scholar] [CrossRef]
- Hu, Y.F.; Russo, I.H.; Russo, J. Estrogen and Human Breast Cancer. In Endocrine Disruptors; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–25. [Google Scholar]
- Li, X.; Yang, J.; Peng, L.; Sahin, A.A.; Huo, L.; Ward, K.C.; O’Regan, R.; Torres, M.A.; Meisel, J.L. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat. 2017, 161, 279–287. [Google Scholar] [CrossRef]
- Plottel, C.S.; Blaser, M.J. Microbiome and malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor–Positive Female Breast Cancer. Gynecol. Oncol. 2016, 108, djw029. [Google Scholar]
- Shapira, I.; Sultan, K.; Lee, A.; Taioli, E. Evolving Concepts: How Diet and the Intestinal Microbiome Act as Modulators of Breast Malignancy. ISRN Oncol. 2013, 2013, 693920. Available online: https://pubmed.ncbi.nlm.nih.gov/24187630/ (accessed on 25 September 2013). [CrossRef] [Green Version]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Modi, S.R.; Collins, J.J.; Relman, D.A. Antibiotics and the gut microbiota. J. Clin. Investig. 2014, 124, 4212–4218. [Google Scholar] [CrossRef] [Green Version]
- Velicer, C.M.; Heckbert, S.R.; Lampe, J.W.; Potter, J.D.; Robertson, C.A.; Taplin, S.H. Antibiotic Use in Relation to the Risk of Breast Cancer. JAMA 2004, 291, 827–835. Available online: https://pubmed.ncbi.nlm.nih.gov/14970061/ (accessed on 18 February 2004). [CrossRef] [PubMed] [Green Version]
- Keum, N.; Greenwood, D.C.; Lee, D.H.; Kim, R.; Aune, D.; Ju, W.; Hu, F.B.; Giovannucci, E.L. Adult weight gain and adiposity-related cancers: A dose-response meta-analysis of prospective observational studies. J. Natl. Cancer Inst. 2015, 107, djv088. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705–721. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4134513/ (accessed on 14 August 2015). [CrossRef] [Green Version]
- Hanafi, N.I.; Mohamed, A.S.; Kadir, S.H.S.A.; Othman, M.H.D. Overview of Bile Acids Signaling and Perspective on the Signal of Ursodeoxycholic Acid, the Most Hydrophilic Bile Acid, in the Heart. Biomolecules 2018, 8, 159. [Google Scholar] [CrossRef] [Green Version]
- Mikó, E.; Vida, A.; Kovács, T.; Ujlaki, G.; Trencsényi, G.; Márton, J.; Sári, Z.; Kovács, P.; Boratkó, A.; Hujber, Z.; et al. Lithocholic acid, a bacterial metabolite reduces breast cancer cell proliferation and aggressiveness. Biochim. Et Biophys. Acta (BBA)—Bioenerg. 2018, 1859, 958–974. [Google Scholar] [CrossRef] [PubMed]
- Mikó, E.; Kovács, T.; Sebő, É.; Tóth, J.; Csonka, T.; Ujlaki, G.; Kovács, T.; Szabó, J.; Méhes, G.; Bai, P. Microbiome-Microbial Metabolome-Cancer Cell Interactions in Breast Cancer-Familiar, but Unexplored. Cells 2019, 8, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampsell, K.; Hao, D.; Reimer, R.A. The Gut Microbiota: A Potential Gateway to Improved Health Outcomes in Breast Cancer Treatment and Survivorship. Int. J. Mol. Sci. 2020, 21, 9239. [Google Scholar] [CrossRef] [PubMed]
- Kovács, P.; Csonka, T.; Kovács, T.; Sári, Z.; Ujlaki, G.; Sipos, A.; Karányi, Z.; Szeőcs, D.; Hegedűs, C.; Uray, K.; et al. Lithocholic Acid, a Metabolite of the Microbiome, Increases Oxidative Stress in Breast Cancer. Cancers 2019, 11, 1255. Available online: https://pubmed.ncbi.nlm.nih.gov/31461945/ (accessed on 27 August 2019). [CrossRef] [PubMed] [Green Version]
- Jaye, K.; Li, C.G.; Chang, D.; Bhuyan, D.J. The role of key gut microbial metabolites in the development and treatment of cancer. Gut Microbes 2022, 14, 2038865. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef] [Green Version]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Kalampokas, E.; Kalampokas, T.; Spartalis, E.; Daskalopoulou, A.; Valsami, S.; Kontos, M.; Nonni, A.; et al. Histone Deacetylases as New Therapeutic Targets in Triple-negative Breast Cancer: Progress and Promises. Cancer Genom. Proteom. 2017, 14, 299–313. [Google Scholar]
- Rodrigues, M.F.; Carvalho, É.; Pezzuto, P.; Rumjanek, F.D.; Amoêdo, N.D. Reciprocal modulation of histone deacetylase in-hibitors sodium butyrate and trichostatin A on the energy metabolism of breast cancer cells. J. Cell. Biochem. 2015, 116, 797–808. [Google Scholar] [CrossRef]
- Salimi, V.; Shabani, M.; Nourbakhsh, M.; Tavakoli-Yaraki, M. Involvement of 15-lipoxygenase-1 in the regulation of breast cancer cell death induced by sodium butyrate. Cytotechnology 2016, 68, 2519–2528. [Google Scholar] [CrossRef] [Green Version]
- Semaan, J.; El-Hakim, S.; Ibrahim, J.-N.; Safi, R.; Elnar, A.A.; El Boustany, C. Comparative effect of sodium butyrate and sodium propionate on proliferation, cell cycle and apoptosis in human breast cancer cells MCF-7. Breast Cancer 2020, 27, 696–705. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, P.-C.; Ma, Y.-B.; Fan, R.; Gao, F.-F.; Zhang, J.-W.; Wei, L. Sodium butyrate-induced apoptosis and ultrastructural changes in MCF-7 breast cancer cells. Ultrastruct. Pathol. 2016, 40, 200–204. [Google Scholar] [CrossRef]
- Guan, X.; Chen, W.; Wei, F.; Xu, J.; Wang, Y.; Chen, L.; Wang, J. Trastuzumab Enhances the Anti-Tumor Effects of the Histone Deacetylase Inhibitor Sodium Butyrate on a HER2-Overexpressing Breast Cancer cell line. Int. J. Mol. Med. 2011, 28, 985–991. Available online: https://www.spandidos-publications.com/10.3892/ijmm.2011.790 (accessed on 1 September 2011). [CrossRef]
- Jiang, W.; Guo, Q.; Wu, J.; Guo, B.; Wang, Y.; Zhao, S.; Lou, H.; Yu, X.; Mei, X.; Wu, C.; et al. Dual effects of sodium butyrate on hepatocellular carcinoma cells. Mol. Biol. Rep. 2012, 39, 6235–6242. [Google Scholar] [CrossRef]
- Zheng, H.-H.; Du, C.-T.; Yu, C.; Tang, X.-Y.; Huang, R.-L.; Zhang, Y.-Z.; Gao, W.; Xie, G.-H. The Relationship of Tumor Microbiome and Oral Bacteria and Intestinal Dysbiosis in Canine Mammary Tumor. Int. J. Mol. Sci. 2022, 23, 10928. [Google Scholar] [CrossRef]
- Zhao, K.; Hu, Y. Microbiome Harbored within Tumors: A New Chance to Revisit Our Understanding of Cancer Pathogenesis and Treatment. Signal Transduct. Target. Ther. 2020, 5, 136. Available online: https://www.nature.com/articles/s41392-020-00244-1 (accessed on 29 July 2020). [CrossRef] [PubMed]
- Xuan, C.; Shamonki, J.M.; Chung, A.; DiNome, M.L.; Chung, M.; Sieling, P.A.; Lee, D.J. Microbial Dysbiosis Is Associated with Human Breast Cancer. PLoS ONE 2014, 9, e83744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Z.; Ye, Z.; Zhu, P.; Zhu, J.; Fang, S.; Qiu, T.; Li, Y.; Meng, L. New Developments and Opportunities of Microbiota in Treating Breast Cancers. Front. Microbiol. 2022, 13, 818793. [Google Scholar] [CrossRef]
- Thu, M.S.; Chotirosniramit, K.; Nopsopon, T.; Hirankarn, N.; Pongpirul, K. Human gut, breast, and oral microbiome in breast cancer: A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1144021. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Magno, S.; Albanese, D.; Donati, C.; Molinari, R.; Filippone, A.; Masetti, R.; Merendino, N. Characterization of human breast tissue microbiota from core needle biopsies through the analysis of multi hypervariable 16S-rRNA gene regions. Sci. Rep. 2018, 8, 16893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Wei, Z.; Tan, F.; Peck, K.N.; Shih, N.; Feldman, M.; Rebbeck, T.R.; Alwine, J.C.; Robertson, E.S. Distinct Microbiological Signatures Associated with Triple Negative Breast Cancer. Sci. Rep. 2015, 5, 15162. Available online: https://www.nature.com/articles/srep15162/ (accessed on 15 October 2015). [CrossRef] [PubMed] [Green Version]
- Smith, A.; Pierre, J.F.; Makowski, L.; Tolley, E.; Lyn-Cook, B.; Lu, L.; Vidal, G.; Starlard-Davenport, A. Distinct Microbial Communities that Differ by Race, Stage, or Breast-Tumor Subtype in Breast Tissues of non-Hispanic Black and non-Hispanic White Women. Sci. Rep. 2019, 9, 11940. Available online: https://www.nature.com/articles/s41598-019-48348-1 (accessed on 16 August 2019). [CrossRef] [Green Version]
- Thompson, K.J.; Ingle, J.N.; Tang, X.; Chia, N.; Jeraldo, P.R.; Walther-Antonio, M.; Kandimalla, K.K.; Johnson, S.; Yao, J.Z.; Harrington, J.; et al. A comprehensive analysis of breast cancer microbiota and host gene expression. PLoS ONE 2017, 12, e0188873. [Google Scholar] [CrossRef]
- Parida, S.; Wu, S.; Siddharth, S.; Wang, G.; Muniraj, N.; Nagalingam, A.; Hum, C.; Mistriotis, P.; Hao, H.; Talbot, C.C.; et al. Data from A Procarcinogenic Colon Microbe Promotes Breast Tumorigenesis and Metastatic Progression and Concomitantly Activates Notch and β-Catenin Axes. Cancer Discov. 2021, 11, 1138–1157. Available online: https://pubmed.ncbi.nlm.nih.gov/33408241/ (accessed on 6 January 2021). [CrossRef]
- Banerjee, S.; Wei, Z.; Tian, T.; Bose, D.; Shih, N.N.C.; Feldman, M.D.; Khoury, T.; De Michele, A. Prognostic correlations with the microbiome of breast cancer subtypes. Cell Death Dis. 2021, 12, 831. [Google Scholar] [CrossRef]
- Tzeng, A.; Sangwan, N.; Jia, M.; Liu, C.C.; Keslar, K.S.; Downs-Kelly, E.; Fairchild, R.L.; Al-Hilli, Z.; Grobmyer, S.R.; Eng, C. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast Cancer Colonization by Fusobacterium nucleatum Accelerates Tumor Growth and Metastatic Progression. Nat. Commun. 2020, 11, 3259. Available online: https://www.nature.com/articles/s41467-020-16967-2 (accessed on 26 June 2020). [CrossRef] [PubMed]
- Nougayrède, J.-P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J.; Dobrindt, U.; Oswald, E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ramos, G.; Petit, C.R.; Marcq, I.; Boury, M.; Oswald, E.; Nougayrède, J.-P. Escherichia coli Induces DNA Damage In Vivo and Triggers Genomic Instability in Mammalian Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 11537–11542. Available online: https://pubmed.ncbi.nlm.nih.gov/20534522/ (accessed on 7 June 2010). [CrossRef] [PubMed]
- Le Noci, V.; Bernardo, G.; Bianchi, F.; Tagliabue, E.; Sommariva, M.; Sfondrini, L. Toll Like Receptors as Sensors of the Tumor Microbial Dysbiosis: Implications in Cancer Progression. Front. Cell Dev. Biol. 2021, 9. Available online: https://www.frontiersin.org/articles/10.3389/fcell.2021.732192/full (accessed on 17 September 2021). [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Weng, W.; Guo, B.; Cai, G.; Ma, Y.; Cai, S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 14. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Volk-Draper, L.D.; Ran, S. TLR4 is a novel determinant of the response to paclitaxel in breast cancer. Mol. Cancer Ther. 2013, 12, 1676–1687. [Google Scholar] [CrossRef] [Green Version]
- Magrini, E.; Di Marco, S.; Mapelli, S.N.; Perucchini, C.; Pasqualini, F.; Donato, A.; de la Luz Guevara Lopez, M.; Carriero, R.; Ponzetta, A.; Colombo, P.; et al. Complement activation promoted by the lectin pathway mediates C3aR-dependent sarcoma progression and immunosuppression. Nat. Cancer 2021, 2, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, Y.; Tupin, E.; Wu, D.; Fujio, M.; Garcia-Navarro, R.; Benhnia, M.R.-E.-I.; Zajonc, M.; Ben-Menachem, G.; Ainge, G.; Painter, G.F.; et al. Faculty Opinions Recommendation of Natural Killer T Cells Recognize Diacylglycerol Antigens from Pathogenic Bacteria. Nat. Immunol. 2006, 7, 978–986. Available online: https://www.nature.com/articles/ni1380 (accessed on 1 January 2011). [CrossRef]
- Hix, L.M.; Shi, Y.H.; Brutkiewicz, R.R.; Stein, P.L.; Wang, C.-R.; Zhang, M. CD1d-Expressing Breast Cancer Cells Modulate NKT Cell-Mediated Antitumor Immunity in a Murine Model of Breast Cancer Metastasis. PLoS ONE 2011, 6, e20702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojanotko-Harri, A.; Nikkari, T.; Harrl, M.-P.; Paunio, K. Metabolism of progesterone and testosterone by Bacillus cereus strain Socransky 67 and Streptococcus mutans strain Ingbritt. Oral Microbiol. Immunol. 1990, 5, 237–239. [Google Scholar] [CrossRef]
- Wiebe, J.P.; Muzia, D.; Hu, J.; Szwajcer, D.; Hill, S.A.; Seachrist, J.L. The 4-pregnene and 5alpha-pregnane progesterone metabolites formed in non tumorous and tumorous breast tissue have opposite effects on breast cell proliferation and adhesion. Cancer Res. 2000, 60, 936–943. [Google Scholar] [PubMed]
- Wang, H.; Rong, X.; Zhao, G.; Zhou, Y.; Xiao, Y.; Ma, D.; Jin, X.; Wu, Y.; Yan, Y.; Yang, H.; et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 2022, 34, 581–594.e8. [Google Scholar] [CrossRef]
- Arroyo, R.; Martín, V.; Maldonado, A.; Jiménez, E.; Fernández, L.; Rodríguez, J.M. Treatment of infectious mastitis during lactation: Antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010, 50, 1551–1558. [Google Scholar] [CrossRef]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.-P.; Ricciardi-Castagnoli, P. Dendritic Cells Express Tight Junction Proteins and Penetrate Gut Epithelial Monolayers to Sample Bacteria. Nat. Immunol. 2001, 2, 361–367. Available online: https://pubmed.ncbi.nlm.nih.gov/11276208/ (accessed on 26 February 2001). [CrossRef]


| Breast Cancer Tissue | Bacteria | Molecular Mechanism | Reference |
|---|---|---|---|
| Human | Escherichia coli and Staphylococcus | Induction of DNA double-strand break and genomic instability in vitro | [14] |
| Human | Clostridiales | Inhibition of tumor growth by producing the metabolite trimethylamine N-oxide (TMAO) that activates CD8+ T cells- mediated antitumor-immunity | [53] |
| Human | Fusobacterium nucleatum | Breast tumor progression and metastases by fap-2 dependent binding of the bacterium to breast cancer tissue Gal-GalNac | [68] |
| Mice | Staphylococcus, Lactobacillus and Streptococcus | Breast tumor lung metastases by modulating the stress response and influencing cancer cell viability, altering the cell cytoskeleton | [15] |
| Mice | Staphylococcus epidermidis | Increased T regulatory cell infiltration in the tumor and complement pathway activation in vivo, and increased pro-tumoral M2 macrophages phenotype in vitro | [16] |
| Mice | Micrococcus luteus | Reduction of mammary tumor growth in vivo, and increased anti-tumoral M1 macrophage phenotype in vitro | [16] |
| Mice | Bacteroides fragilis | Breast tumor progression and metastasis through the secretion of the B. fragilis toxin (BFT) | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardo, G.; Le Noci, V.; Di Modica, M.; Montanari, E.; Triulzi, T.; Pupa, S.M.; Tagliabue, E.; Sommariva, M.; Sfondrini, L. The Emerging Role of the Microbiota in Breast Cancer Progression. Cells 2023, 12, 1945. https://doi.org/10.3390/cells12151945
Bernardo G, Le Noci V, Di Modica M, Montanari E, Triulzi T, Pupa SM, Tagliabue E, Sommariva M, Sfondrini L. The Emerging Role of the Microbiota in Breast Cancer Progression. Cells. 2023; 12(15):1945. https://doi.org/10.3390/cells12151945
Chicago/Turabian StyleBernardo, Giancarla, Valentino Le Noci, Martina Di Modica, Elena Montanari, Tiziana Triulzi, Serenella M. Pupa, Elda Tagliabue, Michele Sommariva, and Lucia Sfondrini. 2023. "The Emerging Role of the Microbiota in Breast Cancer Progression" Cells 12, no. 15: 1945. https://doi.org/10.3390/cells12151945
APA StyleBernardo, G., Le Noci, V., Di Modica, M., Montanari, E., Triulzi, T., Pupa, S. M., Tagliabue, E., Sommariva, M., & Sfondrini, L. (2023). The Emerging Role of the Microbiota in Breast Cancer Progression. Cells, 12(15), 1945. https://doi.org/10.3390/cells12151945