Effect of Acute Enriched Environment Exposure on Brain Oscillations and Activation of the Translation Initiation Factor 4E-BPs at Synapses across Wakefulness and Sleep in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
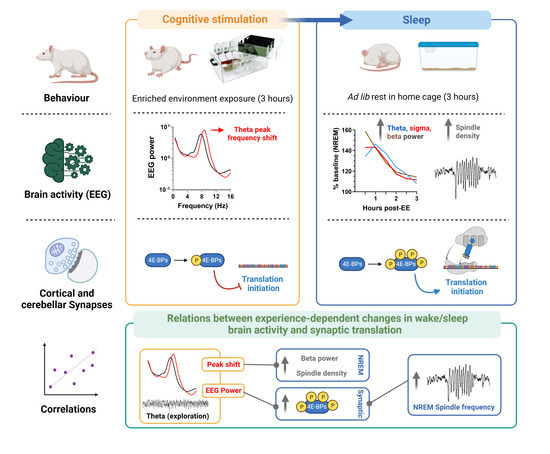
2.2. Experimental Design
2.3. Tissue Collection and Synaptoneurosome Preparation
2.4. Western Blotting and CIP Treatment
2.5. EEG Surgery, Recording and Analysis
2.6. Automated Detection of Spindles
2.7. Statistics
3. Results
3.1. EE Exposure Affects EEGs during Active Wakefulness
3.2. Effect of EE Exposure on Subsequent Sleep–Wakefulness Architecture and EEGs
3.3. Effect of Experience and Sleep on 4E-BP Phosphorylation
3.4. Relation between EEG and Translation Changes
4. Discussion
4.1. Sleep following EE Enhances Conversion of 4E-BPs to Hyperphosphorylated State at Synapses
4.2. Effect of Enriched Environment on Brain Activity across Wakefulness and Sleep
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Blumberg, M.S.; Dooley, J.C.; Tiriac, A. Sleep, Plasticity, and Sensory Neurodevelopment. Neuron 2022, 110, 3230–3242. [Google Scholar] [CrossRef]
- Durkin, J.M.; Aton, S.J. How Sleep Shapes Thalamocortical Circuit Function in the Visual System. Annu. Rev. Vis. Sci. 2019, 5, 295–315. [Google Scholar] [CrossRef]
- Frank, M.G. Sleep and Synaptic Plasticity in the Developing and Adult Brain. Curr. Top. Behav. Neurosci. 2014, 25, 123–149. [Google Scholar] [CrossRef]
- Diering, G.H. Remembering and Forgetting in Sleep: Selective Synaptic Plasticity during Sleep Driven by Scaling Factors Homer1a and Arc. Neurobiol. Stress 2023, 22, 100512. [Google Scholar] [CrossRef]
- Seibt, J.; Frank, M.G. Primed to Sleep: The Dynamics of Synaptic Plasticity Across Brain States. Front. Syst. Neurosci. 2019, 13, 2. [Google Scholar] [CrossRef]
- Sigl-Glöckner, J.; Seibt, J. Peeking into the Sleeping Brain: Using in Vivo Imaging in Rodents to Understand the Relationship between Sleep and Cognition. J. Neurosci. Methods 2019, 316, 71–82. [Google Scholar] [CrossRef]
- Davis, H.P.; Squire, L.R. Protein Synthesis and Memory: A Review. Psychol. Bull. 1984, 96, 518–559. [Google Scholar] [CrossRef]
- Hernandez, P.J.; Abel, T. The Role of Protein Synthesis in Memory Consolidation: Progress amid Decades of Debate. Neurobiol. Learn. Mem. 2008, 89, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.; Stryker, M.P. Rapid Ocular Dominance Plasticity Requires Cortical but Not Geniculate Protein Synthesis. Neuron 2002, 34, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Sun, Y.; Nakamura, R.K.; Mori, K.; Ito, M.; Suda, S.; Namba, H.; Storch, F.I.; Dang, T.P.; Mendelson, W.; et al. Positive Correlations between Cerebral Protein Synthesis Rates and Deep Sleep in Macaca Mulatta. Eur. J. Neurosci. 1997, 9, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ramm, P.; Smith, C.T. Rates of Cerebral Protein Synthesis Are Linked to Slow Wave Sleep in the Rat. Physiol. Behav. 1990, 48, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, C.; Gutierrez, C.M.; Tononi, G. Extensive and Divergent Effects of Sleep and Wakefulness on Brain Gene Expression. Neuron 2004, 41, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, M.; Shockley, K.R.; Romer, M.A.; Galante, R.J.; Zimmerman, J.E.; Naidoo, N.; Baldwin, D.A.; Jensen, S.T.; Churchill, G.A.; Pack, A.I. Macromolecule Biosynthesis: A Key Function of Sleep. Physiol. Genom. 2007, 31, 441–457. [Google Scholar] [CrossRef]
- Vecsey, C.G.; Peixoto, L.; Choi, J.H.K.; Wimmer, M.; Jaganath, D.; Hernandez, P.J.; Blackwell, J.; Meda, K.; Park, A.J.; Hannenhalli, S.; et al. Genomic Analysis of Sleep Deprivation Reveals Translational Regulation in the Hippocampus. Physiol. Genom. 2012, 44, 981–991. [Google Scholar] [CrossRef]
- Seibt, J.; Dumoulin, M.C.; Aton, S.J.; Coleman, T.; Watson, A.; Naidoo, N.; Frank, M.G. Protein Synthesis during Sleep Consolidates Cortical Plasticity in Vivo. Curr. Biol. 2012, 22, 676–682. [Google Scholar] [CrossRef]
- Tudor, J.C.; Davis, E.J.; Peixoto, L.; Wimmer, M.E.; van Tilborg, E.; Park, A.J.; Poplawski, S.G.; Chung, C.W.; Havekes, R.; Huang, J.; et al. Sleep Deprivation Impairs Memory by Attenuating MTORC1-Dependent Protein Synthesis. Sci. Signal. 2016, 9, e13424. [Google Scholar] [CrossRef]
- Banko, J.L.; Klann, E. Cap-Dependent Translation Initiation and Memory. Prog. Brain Res. 2008, 169, 59–80. [Google Scholar] [CrossRef]
- Gingras, A.-C.; Gygi, S.P.; Raught, B.; Polakiewicz, R.D.; Abraham, R.T.; Hoekstra, M.F.; Aebersold, R.; Sonenberg, N. Regulation of 4E-BP1 Phosphorylation: A Novel Two-Step Mechanism. Genes Dev. 1999, 13, 1422–1437. [Google Scholar] [CrossRef] [PubMed]
- Pause, A.; Belsham, G.J.; Gingras, A.C.; Donzé, O.; Lin, T.A.; Lawrence, J.C.; Sonenberg, N. Insulin-Dependent Stimulation of Protein Synthesis by Phosphorylation of a Regulator of 5′-Cap Function. Nature 1994, 371, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Poulin, F.; Gingras, A.C.; Olsen, H.; Chevalier, S.; Sonenberg, N. 4E-BP3, a New Member of the Eukaryotic Initiation Factor 4E-Binding Protein Family. J. Biol. Chem. 1998, 273, 14002–14007. [Google Scholar] [CrossRef] [PubMed]
- Tsukiyama-Kohara, K.; Poulin, F.; Kohara, M.; DeMaria, C.T.; Cheng, A.; Wu, Z.; Gingras, A.C.; Katsume, A.; Elchebly, M.; Spiegelman, B.M.; et al. Adipose Tissue Reduction in Mice Lacking the Translational Inhibitor 4E-BP1. Nat. Med. 2001, 7, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Banko, J.L.; Poulin, F.; Hou, L.; DeMaria, C.T.; Sonenberg, N.; Klann, E. The Translation Repressor 4E-BP2 Is Critical for EIF4F Complex Formation, Synaptic Plasticity, and Memory in the Hippocampus. J. Neurosci. 2005, 25, 9581–9590. [Google Scholar] [CrossRef]
- Bidinosti, M.; Ran, I.; Sanchez-Carbente, M.R.; Martineau, Y.; Gingras, A.-C.; Gkogkas, C.; Raught, B.; Bramham, C.R.; Sossin, W.S.; Costa-Mattioli, M.; et al. Postnatal Deamidation of 4E-BP2 in Brain Enhances Its Association with Raptor and Alters Kinetics of Excitatory Synaptic Transmission. Mol. Cell 2010, 37, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, M.I.; Hernández-Jiménez, M.; Martín, M.E.; Salinas, M.; Alcázar, A. New Hierarchical Phosphorylation Pathway of the Translational Repressor EIF4E-Binding Protein 1 (4E-BP1) in Ischemia-Reperfusion Stress. J. Biol. Chem. 2010, 285, 34355–34363. [Google Scholar] [CrossRef]
- Burnett, P.E.; Barrow, R.K.; Cohen, N.A.; Snyder, S.H.; Sabatini, D.M. RAFT1 Phosphorylation of the Translational Regulators P70 S6 Kinase and 4E-BP1. Proc. Natl. Acad. Sci. USA 1998, 95, 1432–1437. [Google Scholar] [CrossRef]
- Livingstone, M.; Bidinosti, M. Rapamycin-Insensitive MTORC1 Activity Controls EIF4E:4E-BP1 Binding. F1000Res. 2012, 1, 4. [Google Scholar] [CrossRef]
- Mothe-Satney, I.; Yang, D.; Fadden, P.; Haystead, T.A.J.; Lawrence, J.C. Multiple Mechanisms Control Phosphorylation of PHAS-I in Five (S/T)P Sites That Govern Translational Repression. Mol. Cell. Biol. 2000, 20, 3558–3567. [Google Scholar] [CrossRef]
- Banko, J.L.; Hou, L.; Poulin, F.; Sonenberg, N.; Klann, E. Regulation of Eukaryotic Initiation Factor 4E by Converging Signaling Pathways during Metabotropic Glutamate Receptor-Dependent Long-Term Depression. J. Neurosci. 2006, 26, 2167–2173. [Google Scholar] [CrossRef]
- Carroll, M.; Dyer, J.; Sossin, W.S. Serotonin Increases Phosphorylation of Synaptic 4EBP through TOR, but Eukaryotic Initiation Factor 4E Levels Do Not Limit Somatic Cap-Dependent Translation in Aplysia Neurons. Mol. Cell. Biol. 2006, 26, 8586–8598. [Google Scholar] [CrossRef] [PubMed]
- Cajigas, I.J.; Will, T.; Schuman, E.M. Protein Homeostasis and Synaptic Plasticity. EMBO J. 2010, 29, 2746–2752. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.A.; Schuman, E.M. Dendritic Protein Synthesis, Synaptic Plasticity, and Memory. Cell 2006, 127, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.-I.; Horiike, Y.; Matsuzaki, M.; Miyazaki, T.; Ellis-Davies, G.C.R.; Kasai, H. Protein Synthesis and Neurotrophin-Dependent Structural Plasticity of Single Dendritic Spines. Science 2008, 319, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Fares, R.P.; Belmeguenai, A.; Sanchez, P.E.; Kouchi, H.Y.; Bodennec, J.; Morales, A.; Georges, B.; Bonnet, C.; Bouvard, S.; Sloviter, R.S.; et al. Standardized Environmental Enrichment Supports Enhanced Brain Plasticity in Healthy Rats and Prevents Cognitive Impairment in Epileptic Rats. PLoS ONE 2013, 8, e53888. [Google Scholar] [CrossRef] [PubMed]
- Löwel, S.; Kalogeraki, E.; Dehmel, S.; Makowiecki, K. Environmental conditions strongly affect brain plasticity. e-Neuroforum 2018, 24, A19–A29. [Google Scholar] [CrossRef]
- Villasana, L.E.; Klann, E.; Tejada-Simon, M.V. Rapid Isolation of Synaptoneurosomes and Postsynaptic Densities from Adult Mouse Hippocampus. J. Neurosci. Methods 2006, 158, 30–36. [Google Scholar] [CrossRef]
- Seibt, J.; Richard, C.J.; Sigl-Glöckner, J.; Takahashi, N.; Kaplan, D.I.; Doron, G.; de Limoges, D.; Bocklisch, C.; Larkum, M.E. Cortical Dendritic Activity Correlates with Spindle-Rich Oscillations during Sleep in Rodents. Nat. Commun. 2017, 8, 684. [Google Scholar] [CrossRef]
- Phillips, K.G.; Bartsch, U.; McCarthy, A.P.; Edgar, D.M.; Tricklebank, M.D.; Wafford, K.A.; Jones, M.W. Decoupling of Sleep-Dependent Cortical and Hippocampal Interactions in a Neurodevelopmental Model of Schizophrenia. Neuron 2012, 76, 526–533. [Google Scholar] [CrossRef]
- Havekes, R.; Aton, S.J. Impacts of Sleep Loss versus Waking Experience on Brain Plasticity: Parallel or Orthogonal? Trends Neurosci. 2020, 43, 385–393. [Google Scholar] [CrossRef]
- Alwis, D.S.; Rajan, R. Environmental Enrichment and the Sensory Brain: The Role of Enrichment in Remediating Brain Injury. Front. Syst. Neurosci. 2014, 8, 156. [Google Scholar] [CrossRef]
- Bennett, E.L.; Diamond, M.C.; Krech, D.; Rosenzweig, M.R. Chemical and anatomical plasticity brain. Science 1964, 146, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Gelfo, F.; Petrosini, L. Environmental Enrichment Enhances Cerebellar Compensation and Develops Cerebellar Reserve. Int. J. Environ. Res. Public Health 2022, 19, 5697. [Google Scholar] [CrossRef]
- van Praag, H.; Kempermann, G.; Gage, F.H. Neural Consequences of Environmental Enrichment. Nat. Rev. Neurosci. 2000, 1, 191–198. [Google Scholar] [CrossRef]
- Carlqvist, H.; Nikulin, V.V.; Strömberg, J.O.; Brismar, T. Amplitude and Phase Relationship between Alpha and Beta Oscillations in the Human Electroencephalogram. Med. Biol. Eng. Comput. 2005, 43, 599–607. [Google Scholar] [CrossRef]
- Fernandez, L.M.J.; Lüthi, A. Sleep Spindles: Mechanisms and Functions. Physiol. Rev. 2020, 100, 805–868. [Google Scholar] [CrossRef]
- Kirkcaldie, M.T. Neocortex. In The Mouse Nervous System; Watson, C., Paxinos, G., Puelles, L., Eds.; Elsevier: San Diego, CA, USA, 2012; pp. 52–111. [Google Scholar]
- Saraf, A.; Luo, J.; Morris, D.R.; Storm, D.R. Phosphorylation of Eukaryotic Translation Initiation Factor 4E and Eukaryotic Translation Initiation Factor 4E-Binding Protein (4EBP) and Their Upstream Signaling Components Undergo Diurnal Oscillation in the Mouse Hippocampus: Implications for Memory Persistence. J. Biol. Chem. 2014, 289, 20129–20138. [Google Scholar] [CrossRef]
- Boyce, R.; Glasgow, S.D.; Williams, S.; Adamantidis, A. Causal Evidence for the Role of REM Sleep Theta Rhythm in Contextual Memory Consolidation. Science 2016, 352, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Siegle, J.H.; Ritt, J.T.; Ting, J.T.; Feng, G.; Moore, C.I. Selective Optical Drive of Thalamic Reticular Nucleus Generates Thalamic Bursts and Cortical Spindles. Nat. Neurosci. 2011, 14, 1118–1120. [Google Scholar] [CrossRef]
- Noya, S.B.; Colameo, D.; Brüning, F.; Spinnler, A.; Mircsof, D.; Opitz, L.; Mann, M.; Tyagarajan, S.K.; Robles, M.S.; Brown, S.A. The Forebrain Synaptic Transcriptome Is Organized by Clocks but Its Proteome Is Driven by Sleep. Science 2019, 366, eaav2642. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Cirelli, C. Sleep and Synaptic Homeostasis: A Hypothesis. Brain Res. Bull. 2003, 62, 143–150. [Google Scholar] [CrossRef]
- Franken, P.; Dijk, D.J.; Tobler, I.; Borbély, A.A. Sleep Deprivation in Rats: Effects on EEG Power Spectra, Vigilance States, and Cortical Temperature. Am. J. Physiol. 1991, 261, R198–R208. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Deboer, T.; Tobler, I. Topography of EEG Dynamics after Sleep Deprivation in Mice. J. Neurophysiol. 2000, 84, 1888–1893. [Google Scholar] [CrossRef]
- Peyrache, A.; Seibt, J. A Mechanism for Learning with Sleep Spindles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190230. [Google Scholar] [CrossRef]
- Cox, R.; van Driel, J.; de Boer, M.; Talamini, L.M. Slow Oscillations during Sleep Coordinate Interregional Communication in Cortical Networks. J. Neurosci. 2014, 34, 16890–16901. [Google Scholar] [CrossRef]
- Mölle, M.; Marshall, L.; Gais, S.; Born, J. Grouping of Spindle Activity during Slow Oscillations in Human Non-Rapid Eye Movement Sleep. J. Neurosci. 2002, 22, 10941–10947. [Google Scholar] [CrossRef]
- Weber, F.D.; Supp, G.G.; Klinzing, J.G.; Mölle, M.; Engel, A.K.; Born, J. Coupling of Gamma Band Activity to Sleep Spindle Oscillations—A Combined EEG/MEG Study. NeuroImage 2021, 224, 117452. [Google Scholar] [CrossRef]
- Grønli, J.; Rempe, M.J.; Clegern, W.C.; Schmidt, M.; Wisor, J.P. Beta EEG Reflects Sensory Processing in Active Wakefulness and Homeostatic Sleep Drive in Quiet Wakefulness. J. Sleep Res. 2016, 25, 257–268. [Google Scholar] [CrossRef]
- Huber, R.; Deboer, T.; Tobler, I. Effects of Sleep Deprivation on Sleep and Sleep EEG in Three Mouse Strains: Empirical Data and Simulations. Brain Res. 2000, 857, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Korotkova, T.; Ponomarenko, A.; Monaghan, C.K.; Poulter, S.L.; Cacucci, F.; Wills, T.; Hasselmo, M.E.; Lever, C. Reconciling the Different Faces of Hippocampal Theta: The Role of Theta Oscillations in Cognitive, Emotional and Innate Behaviors. Neurosci. Biobehav. Rev. 2018, 85, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Kropff, E.; Carmichael, J.E.; Moser, E.I.; Moser, M.-B. Frequency of Theta Rhythm Is Controlled by Acceleration, but Not Speed, in Running Rats. Neuron 2021, 109, 1029–1039.e8. [Google Scholar] [CrossRef]
- LabGym: Quantification of User-Defined Animal Behaviors Using Learning-Based Holistic Assessment—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/37056376/ (accessed on 28 August 2023).
- Sossin, W.S.; Costa-Mattioli, M. Translational Control in the Brain in Health and Disease. Cold Spring Harb. Perspect. Biol. 2018, 11, a032912. [Google Scholar] [CrossRef] [PubMed]
- Ingiosi, A.M.; Frank, M.G. Goodnight, Astrocyte: Waking up to Astroglial Mechanisms in Sleep. FEBS J. 2023, 290, 2553–2564. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.; Sriram, K. Neuron-Astrocyte Omnidirectional Signaling in Neurological Health and Disease. Front. Mol. Neurosci. 2023, 16, 1169320. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.L.; Petsidou, E.; Saraogi, P.; Bartsch, U.; Gerber, A.P.; Seibt, J. Effect of Acute Enriched Environment Exposure on Brain Oscillations and Activation of the Translation Initiation Factor 4E-BPs at Synapses across Wakefulness and Sleep in Rats. Cells 2023, 12, 2320. https://doi.org/10.3390/cells12182320
Santos JL, Petsidou E, Saraogi P, Bartsch U, Gerber AP, Seibt J. Effect of Acute Enriched Environment Exposure on Brain Oscillations and Activation of the Translation Initiation Factor 4E-BPs at Synapses across Wakefulness and Sleep in Rats. Cells. 2023; 12(18):2320. https://doi.org/10.3390/cells12182320
Chicago/Turabian StyleSantos, José Lucas, Evlalia Petsidou, Pallavi Saraogi, Ullrich Bartsch, André P. Gerber, and Julie Seibt. 2023. "Effect of Acute Enriched Environment Exposure on Brain Oscillations and Activation of the Translation Initiation Factor 4E-BPs at Synapses across Wakefulness and Sleep in Rats" Cells 12, no. 18: 2320. https://doi.org/10.3390/cells12182320
APA StyleSantos, J. L., Petsidou, E., Saraogi, P., Bartsch, U., Gerber, A. P., & Seibt, J. (2023). Effect of Acute Enriched Environment Exposure on Brain Oscillations and Activation of the Translation Initiation Factor 4E-BPs at Synapses across Wakefulness and Sleep in Rats. Cells, 12(18), 2320. https://doi.org/10.3390/cells12182320