1. Introduction
Sugars are a vital source of energy and essential nutrients for living organisms. However, modifications to the lysine and arginine residues of proteins in vivo, which result in the formation of crosslinks, may significantly change the steric structures of proteins, thereby affecting their activities and physical properties. This process is known as glycation or the Maillard reaction and is divided into two stages: an early-stage reaction that produces Amadori transfer products and a late-stage reaction that results in advanced glycation end-products (AGEs) through oxidation, dehydration, and condensation [
1]. AGEs have been implicated in the pathogenesis of various diseases [
2,
3], such as diabetes, liver disease [
4], atherosclerosis [
5], Alzheimer’s disease, and aging [
6].
AGEs represent a diverse cluster of compounds. For instance, Nε-(carboxymethyl)lysine (CML) and Nε-(carboxyethyl)lysine (CEL) are AGEs formed through reactions with glyoxal (GO), methylglyoxal (MGO), and lysine residues in proteins. AGEs can be broadly classified into exogenously derived dietary AGEs, formed through the heat processing of foods like milk and dairy products [
7,
8], and endogenously generated AGEs that accumulate within the body. The development of diseases due to endogenous AGE accumulation is postulated to involve two pathways: (1) the activation of downstream cellular signaling through the RAGE receptor, inducing oxidative stress and inflammation [
9] and (2) cross-linking with intracellular proteins, disrupting normal physiological functions. Additionally, reports emphasize the AGE interactions with the gut microbiota and their consequential effects [
7].
AGEs are generated in vivo not only from glucose, but also from, for example, metabolic intermediates of glucose, degradation products, and Maillard reaction intermediates [
10,
11]. In the classification spectrum of more than 40 AGEs identified to date [
9], glyceraldehyde (GA) plays a crucial role in the formation of GA-AGEs (GA-derived AGEs) through intricate metabolic pathways [
3]. In fructolysis, fructokinase (FK) phosphorylates fructose (Fru), forming Fru-1-phosphate (F-1-P). The subsequent cleavage by aldolase B yields dihydroxyacetone-phosphate and GA. Concurrently, glycolysis metabolizes GA-3-phosphate (GA-3-P) through GA-3-P dehydrogenase (GAPDH), ultimately leading to pyruvate. Reduced GAPDH activity results in intracellular accumulation of GA-3-P, promoting non-enzymatic reactions that contribute to GA formation. Moreover, under hyperglycemic conditions, the polyol pathway is activated and glucose is reduced by aldose reductase to form sorbitol, which is subsequently oxidized by sorbitol dehydrogenase to form Fru. These excess GAs, arising from impaired glucose metabolism, non-enzymatically react with proteins, ultimately leading to the formation of the GA-AGEs implicated in diseases associated with diabetic complications, insulin resistance, heart disease, Alzheimer’s disease, hypertension, nonalcoholic steatohepatitis, and cancer [
3]. The toxic effects of GA-AGEs and their specific structures that are responsible for toxicity remain unclear. However, an antibody targeting GA-AGEs was shown to effectively mitigate neurotoxicity caused by serum AGEs in patients with diabetic nephropathy undergoing hemodialysis [
12]. Other antibodies targeting different types of AGEs or CML did not exert similar protective effects. These findings indicate that only AGE structures containing epitopes recognized by the anti-GA-AGE antibody are toxic. They are referred to as toxic AGEs (TAGEs) and are distinct from other known GA-AGEs, such as 3-hydroxy-5-hydroxymethyl-pyridinium (GLAP), triosidines, and MG-H1. None of these structures showed AGE-specific fluorescence or protein cross-linking. Two compounds with a 1,4-dihydropyrazine ring that showed fluorescence and had cross-links were identified as TAGE candidate structures (PCT/JP2019/34195).
The late-stage Maillard reaction is irreversible and once GA-AGEs are formed, there are no known enzymes that specifically remove the sites modified by glycation. Glycation modifications have been suggested to compromise cellular homeostasis by inducing the loss of function of various biomolecules or by forming toxic aggregates, which inactivate other normal essential proteins [
3]. The ubiquitin–proteasome pathway and autophagy pathway are intracellular systems that remove defective proteins and aggregates. These two pathways are not independent of each other, and they function cooperatively [
13,
14,
15,
16]. Recent studies suggested that AGEs are closely associated with autophagy [
17]. Their effects on autophagy are complex, with some studies indicating that they inhibit autophagy [
18,
19] and others suggesting that they induce autophagy [
20,
21,
22,
23,
24]. Therefore, the coordinated role of the two intracellular degradation mechanisms in the AGE clearance process remains unclear. Although AGEs are formed and accumulate in proteins with a long turnover due to a decrease in the activities of intracellular protein quality control mechanisms with aging, the mechanisms underlying intracellular GA-AGE degradation and removal have yet to be clarified. A more detailed understanding of these mechanisms is critical for the development of effective therapeutic strategies that mitigate the detrimental effects of GA-AGE accumulation.
In the present study, we found that N-terminal checkpoint kinase 1 cleaved products (CHK1-CPs) and their mimetic protein (d270WT) were susceptible to intracellular degradation upon the administration of GA, and also that a kinase-dead mutant (d270KD) of d270WT exhibited more rapid GA-responsive degradation with the formation of high-molecular-weight complexes (typical features indicative of GA-AGE conversion). d270KD, which is rapidly degraded with the generation of GA-AGEs, may serve as a useful model for GA-AGE formation in future research on the molecular mechanisms that act on the clearance of GA-AGEs that accumulate in cells.
2. Materials and Methods
2.1. Antibodies
The following primary antibodies were used: anti-mouse IgG2a magnetic beads (M076-11), anti-DYKDDDDK (FLAG)-tag magnetic beads (M185-11), anti-V5-tag magnetic beads (M16711), anti-normal rabbit IgG (PM035), and an anti-DYKDDDDK (FLAG)-tag (PM020) for immunoprecipitation, and anti-HA-tag (M132-3), anti-α-tubulin-HRP, and anti-microtubule-associated protein 1 light chain 3 (LC3; M186-3) for Western blotting from Medical & Biological Laboratories (MBL, Nagoya, Japan); anti-phospho-SQSTM1/p62 (Ser403) (#39786), anti-SQSTM1/p62 (#5114), and anti-HUWE1 (#5695) from Cell Signaling Technologies (CST, Beverley, MA, USA); anti-V5-HRP (R961-25) from Thermo Fisher Scientific (Waltham, MA, USA); anti-FLAG-HRP (015-22391) from Wako Pure Chemicals (Osaka, Japan); anti-ubiquitin (#AUB01) from Cytoskeleton (Denver, CO, USA); and anti-SPRTN (HPA025073) from Sigma-Aldrich (St Louis, MO, USA). An anti-GA-AGE (TAGE) antibody was prepared as previously reported [
25]. The anti-TAGE antibody specifically identified unique epitopes other than the known structures derived from GA, such as GLAP and triosidines. However, it did not recognize well-known AGEs containing CML and Nε-(carboxyethyl)lysine (CEL) or bind to other AGEs derived from reducing sugars and carbonyl molecules, including pyrraline, pentosidine, crossline, argpyrimidine, GO- or MGO-lysine dimers, and GO- or MGO-derived hydroimidazolone.
2.2. Cell Culture
COS-7 cells were obtained from the American Type Culture Collection and cultured in Dulbecco’s modified Eagle medium (DMEM + GlutaMax medium; Gibco BRL, Gland Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; JRH Biosciences, Lenexa, KS, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin (both from Gibco BRL) at 37 °C in a humidified atmosphere of 5% CO2. HeLa cells (a gift from Dr. Sumiyo Akazawa, Kanazawa Medical University, Japan) were cultured in minimum essential medium (MEM alpha + GlutaMax medium; Gibco BRL, Waltham, MA, USA) supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2. Cells were subcultured when they reached 70–80% confluence. In experiments where GA (purity 98% or higher; catalog number: 17014-94, Nacalai Tesque, Tokyo, Japan) was applied to cells, GA or phosphate buffer was added at the concentrations indicated. In the aminoguanidine (AG; purity 97% or higher; catalog number: 328-26432, FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan)-induced suppression experiment, AG at the indicated concentrations was added to cells 2 h prior to the treatment with GA. Regarding the proteasome inhibitor treatment, cells were treated with 10 µM MG-132 (Sigma-Aldrich, St. Louis, MO, USA) or DMSO (Nacalai Tesque, Kyoto, Japan) for 6 h. In the autophagy inhibition experiments, 50 µM chloroquine (Sigma-Aldrich) or phosphate buffer was added to HeLa cells 20 h prior to the GA treatment.
2.3. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
LDH released into the culture supernatant was measured using a LDH cytotoxicity assay kit (Nacalai Tesque, Kyoto, Japan) following the manufacturer’s instructions. Briefly, HeLa cells were plated on 24-well plates (5 × 104 cells/well). The cells were stimulated with 4 mM GA or phosphate buffer for the indicated time. The culture medium (100 μL) was collected and mixed with 100 μL of the substrate solution. After an incubation at room temperature for 20 min, 50 μL of the stop solution was added and the absorbance was measured at 490 nm using an iMark™ Microplate Reader (Bio-Rad, Hercules, CA, USA). LDH levels in the cell culture supernatant collected from the GA-treated cell group were normalized relative to the cultured medium collected from the control group (phosphate buffer-treated group).
2.4. Generation of Expression Plasmid Constructs
Flag-tagged CHK1 expression constructs, including the wild-type (WT) form and its kinase-dead mutant form (KDmut), were previously described [
26]. Briefly, cDNA encoding full-length rat Chk1 was amplified from rat cardiac myocyte cDNA using a forward primer containing the Flag-tag sequence at the 5′ end and then subcloned into the pcDNA4/HisMax vector. Xpress- and 6 × His-tag sequences at the N terminus were removed from the vector by PCR using the KOD-plus-mutagenesis kit (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. Deletion at the C terminus and point mutations in Flag-Chk1 expression vectors were created by PCR using the KOD-plus-mutagenesis kit. To generate the d270KD-EGFP-V5 expression construct, full-length EGFP cDNA was amplified by PCR using the pEGFP-C1 vector (BD Biosciences, San Jose, CA, USA) and subcloned into the pcDNA3.1 vector (Thermo Fisher Scientific). The open reading frame of EGFP with the V5-tag and 6 × His-tag sequences at the C terminus was then amplified using the vector as a template by PCR using PrimeSTAR Max DNA polymerase, subcloned into the pENTR/D-TOPO vector (Thermo Fisher Scientific) using the TOPO
® cloning procedure, and then transformed into the mammalian expression vector pDEST30 (Thermo Fisher Scientific) using LR clonase II enzyme. Fragments containing the N-terminal region of Chk1 (amino acids 1-270) in pFlag-Chk1Wt and its KDmut (d270KD) vectors were cloned in the vector in-frame with the gene that encodes the EGFP-V5/6 × His-tag at the 3′ end using the In-Fusion
® HD PCR cloning kit (Takara, Shiga, Japan). To generate the d270KD-ZsGreen expression construct, full-length ZsGreen1 cDNA was amplified by PCR using the ZsGreen1-1 vector (Clontech, Palo Alto, CA, USA) and the fragments were cloned into the vector in-frame with the gene encoding Flag-d270KD at the 3′ end using the In-Fusion
® HD PCR cloning kit (Takara, Shiga, Japan). HA-ubiquitin was a gift from Edward Yeh (Addgene plasmid #18712). All constructs were sequenced to ensure proper ligation in the correct frame and Taq polymerase fidelity using the ABI PRISM TM 310 genetic analyzer (Applied Biosystems, Foster City, CA, USA).
2.5. Transfection
Regarding transient plasmid DNA transfection, cells were seeded on 35, 60, 100 mm, or 6-well tissue culture plates, cultured in complete growth medium, and then transfected using FuGENE® 4K (Promega, Madison, WI, USA) according to the manufacturer’s protocol. The transfection of siRNA was performed using the RNAiMax Transfection reagent (Thermo Fisher Scientific) according to the manufacturer’s recommendations. SignalSilence® siRNA (#6241, Cell Signaling Technologies) targeting Chk1, Silencer Select siRNA (Ambion, Valencia, CA, USA) targeting Huwe1 (siRNA ID: s19596 and s19597), SQSTM1 (siRNA ID: s16962), SPRTN (siRNA ID: s38329), or scrambled siRNA (Silencer Select Negative Control #1, catalogue #4390843) was used. The final siRNA concentration was 50 nM for SignalSilence® siRNAs and 5 nM for Silencer Select siRNAs.
2.6. Western Blot
Transfected COS-7 and HeLa cells were lysed in lysis buffer (CelLytic™M cell lysis reagent; Sigma-Aldrich) containing proteinase inhibitors and phosphatase inhibitors (both from Nacalai Tesque). After the cellular debris was removed by centrifugation, protein concentrations in the supernatants were measured using the Qubit protein assay kit (Thermo Fisher Scientific). To detect GA-AGEs (TAGE) or high-molecular-weight complexes, cells were lysed in RIPA buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% Na deoxycholate, and 0.1% SDS), centrifuged, and separated into supernatant (soluble fraction) and pellet (insoluble fraction). After the addition of SDS sample buffer to each fraction, the RIPA-insoluble fraction was sonicated. All samples were boiled at 95 °C for 5 min, separated on 5–20 or 12.5% SDS-PAGE gels, and analyzed by Western blotting. Target proteins were visualized by Chemi-Lumi One L, Super (Nacalai Tesque), or Immobilon Forte Western HRP Substrate (Millipore, Bedford, MA, USA). A densitometric quantification of the resultant blots was performed using NIH ImageJ software (version 1.54d).
2.7. In Vitro GA-AGE Modification Assay
The in vitro GA-AGE modification of the d270KD protein was performed according to previous methods with some changes. Briefly, HeLa cells transfected with Flag-tag-fused d270KD (Flag-d270KD) were lysed in CelLytic™M cell lysis reagent containing proteinase inhibitors and phosphatase inhibitors. After the cellular debris was removed by centrifugation, total cellular proteins were incubated with anti-Flag antibody-immobilized magnetic beads at 4 °C overnight, and the resulting immunoprecipitates were washed three times with lysis reagent. After the elution of Flag-d270KD with 40 µL of Flag peptide (2 mg/mL), 200 µL of PBS was added. The eluted samples, including Flag-d270KD, were then ultrafiltered and concentrated with Amicon Ultra 0.5 (10 K) (Millipore) to remove the FLAG peptide. This purified Flag-d270KD recombinant protein (2.5 µg) was incubated in 50 µL of PBS for 20 h with or without the addition of 4 mM GA. After the addition of SDS sample buffer to these reaction mixtures, they were boiled at 95 °C for 5 min.
2.8. In Vivo Ubiquitination Assay
For the in vivo ubiquitylation assays, the d270KD-EGFP expression vector d270KD-EGFP was used to transfect COS-7 cells (100 mm plates) for 48 h. The cells were treated with 10 µM MG132 and then harvested after 8 h. The cell pellets were resuspended in denaturing buffer (1.5% SDS, 50 mM Tris–HCl pH 7.5, and 5 mM DTT) followed by boiling for 10 min, and were then diluted 10-fold with CelLytic™M cell lysis reagent containing proteinase inhibitors and phosphatase inhibitors. After the cellular debris was removed by centrifugation, the extracted proteins were immunoprecipitated with anti-V5 antibody-immobilized magnetic beads and subjected to the assay described above. Immunoprecipitates were analyzed by Western blotting with anti-ubiquitin antibodies or anti-V5 antibodies.
2.9. Luciferase Assay
The gene encoding luciferase was amplified by PCR using the pGL4.54 vector (Promega) as a template with the forward primer 5′-ACAAACACACTTAACATGGAAGATGCCAAAAAC-3′ and reverse primer 5′-GTAACAGGCCTTCTACACGGGCGATCTTGCCGCCC-3′. The pFlag-d270KD plasmid vector was amplified by PCR using the forward primer 5′-TA GAAGGGCCTGTACCTAGGATCCAGT-3′ and reverse primer 5′-GTTAAGTGGTTTGTTATACCATCTA-3′ to form a linear strand. The luciferase gene was then inserted downstream of the d270KD gene using the InFusion HD cloning kit (Clontech, Palo Alto, CA, USA). The accuracy of the gene insertion site was confirmed by a sequence analysis. Twenty-four hours after the transfection of the d270KD fusion luciferase expression vector (d270KD-Luc) into cells cultured in 6-well plates, the firefly luciferase reporter assay was performed using the Luciferase Reporter Assay System (Promega Madison, WI, USA). Three independent assays were performed under each condition.
2.10. Fluorescence Imaging Analysis
COS-7 cells were grown in six-well plates containing collagen-coated glass coverslips (diameter of 12 mm, Iwaki, Shizuoka, Japan) and transfected with the d270KD-EGFP expression vector at 60% confluence using FuGENE® 4K as described above. After an incubation for 24 h in complete medium, the cells were treated with or without GA (2 mM) for the indicated time and fixed with ice-cold 4% paraformaldehyde in PBS for 10 min. The fixed cells were washed three times for 5 min each with PBS and then mounted with Prolong gold antifade reagent/DAPI (Thermo Fisher Scientific). All fluorescence images were obtained using a digital high-definition microscope system (BZ-9000, Keyence, Osaka, Japan) with the following filter sets: OP-66834, Ex360/40 Em460/50; OP-66836, Ex470/40 Em535/50; OP-66838, Ex560/40 Em630/60.
2.11. Immunoprecipitation Assay of High-Molecular-Weight Complexes Formed by a GA Stimulation
The d270KD-ZsGreen expression vector was transfected into HeLa cells (100 mm plates) for 48 h. The cells were treated with 2 mM GA and then harvested after 3 h. The cells were lysed in RIPA buffer containing proteinase inhibitors and phosphatase inhibitors, centrifuged, and separated into supernatant (soluble fraction) and pellet (insoluble fraction). After the addition of solubilization buffer containing a high concentration of SDS (2.0% SDS, 50 mM Tris-HCl pH 7.5) to the pellet, the RIPA-insoluble fraction was sonicated. After removing the high concentration of SDS from the fraction using the Pierce™ SDS-PAGE Sample Prep Kit (Thermo Fisher Scientific), ultrafiltration was performed using Amicon Ultra 0.5 (30 K) (Millipore), while the solvent of the fraction was replaced with CelLytic™M cell lysis reagent containing proteinase inhibitors and phosphatase inhibitors. The anti-FLAG antibody (MBL) or normal rabbit IgG (MBL) was added to the soluble and insoluble fractions, which were then incubated at 4 °C overnight. The coupling of the antibodies to Dynabeads Protein A (Thermo Fisher Scientific) was performed by adding CelLytic™M-equilibrated Dynabeads directly to both fractions, followed by an incubation at room temperature for 30 min. Immunoprecipitated Dynabead complexes were washed once with CelLytic™M reagent and three times with TBS-T. Proteins bound to the Dynabead–antibody complexes were eluted by adding 50 µL of the FLAG peptide (2 mg/mL). After the addition of SDS sample buffer to each fraction, all samples were boiled at 95 °C for 5 min, separated on 5–20% SDS-PAGE gels, and analyzed by Western blotting with the indicated antibodies.
2.12. Statistical Analysis
All numerical results are reported as the mean ± SEM of at least three measurements. Statistical analyses were performed using the paired Student’s t-test, a one-way ANOVA followed by Dunnett’s post hoc test, or a two-way ANOVA followed by the Bonferroni post hoc test with GraphPad Prism software (Version 5.0, GraphPad Prism Software, San Diego, CA, USA). Differences were considered to be significant at p < 0.05 and were denoted by an asterisk in the graphs.
4. Discussion
Under hyperglycemic conditions, glucose, fructose, and its metabolic intermediates bind non-enzymatically to intracellular proteins to produce AGEs. Among these AGEs, GA-AGEs, also known as TAGE, are highly cytotoxic and have been implicated in various pathological conditions [
3], such as diabetic complications, insulin resistance, heart disease, Alzheimer’s disease, hypertension, nonalcoholic steatohepatitis, obesity, and cancer. GA-AGEs predominantly form intracellularly as a result of glycation reactions between GA and intracellular proteins, resulting in a wide range of GA-AGE molecules with different sizes and properties. The mechanisms by which GA-AGEs exert their cytotoxic effects have not yet been elucidated in detail; however, previous studies suggested that GA-AGE toxicity was attributed to oxidative stress damage, the loss of protein functions due to glycation modifications, and the aggregation and accumulation of GA-AGEs [
3].
To the best of our knowledge, this is the first study to show the rapid degradation (less than 2 h) of a protein in an experimental model in which intracellular AGEs were formed by the administration of glycating agents, such as GA and methylglyoxal (MGO), to cells. Even when d270KD was fused with relatively stable proteins, such as EGFP, ZsGreen1, or luciferase, the GA-responsive rapid degradation property was retained (
Figure 3,
Figure 4, and
Figure S2), indicating that d270KD functions as a degradation element induced by glycated substances. Furthermore, a reporter assay using luciferase activity in d270KD-Luc-transfected cells allowed the degradation process to be monitored without a Western blot analysis, and GA-induced reductions in luciferase activity were attenuated by inhibitors of AGE formation in a concentration-dependent manner (
Figure 4c). Therefore, this reporter assay using d270KD-Luc-transfected cells may be a useful tool for the rapid screening of natural compounds that inhibit the formation of AGEs.
The etiology of GA-AGE toxicity is attributed to the functional impairment of the proteins themselves as a result of GA-AGE modifications. Previous studies demonstrated that chaperone molecules, such as Hsc70, and a mediator of apoptosis, caspase-3, formed high-molecular-weight complexes that led to a loss of activity due to GA-AGE modifications [
3]. In the present study, we observed the similar formation and accumulation of high-molecular-weight complexes of CHK1 after the GA stimulation, as shown in
Figure 1. CHK1 is a well-known key player in the cellular stress response to DNA damage and the normal progression of the cell cycle under non-stress conditions [
27]. Therefore, CHK1 may be a target of GA-AGE modifications, and its functional impairment may be responsible for the induction of GA-AGE-mediated cell death. Notably, when HeLa cells expressing Flag-CHK1 were stimulated with GA, a decrease in CHK1-CP levels was observed, suggesting that N-terminal CHK1 kinase fragments were susceptible to glycation modifications by GA (
Figure 1b). Consistent with this result, d270WT (a kinase fragment containing the N-terminal 270-amino acid region of wild-type CHK1), which mimics CHK1-CPs, also showed time-dependent proteolysis upon GA treatment, but more slowly than d270KD (
Figure 2a). In the course of our research, d270KD demonstrated accelerated degradation in response to GA compared to d270WT. However, the precise underlying reasons for this phenomenon remain unclear at present. One plausible explanation is that the structural features of d270KD make it more susceptible to ubiquitin modification than its wild-type counterpart, potentially leading to rapid degradation through the proteasome pathway.
As Halder et al. previously reported [
28], CHK1-CPs are formed through cleavage by SPRTN metalloprotease, and in our cell model, the knockdown of SPRTN by the transfection of a specific siRNA reduced the basal expression levels of Flag-CHK1-CPs (
Figure 1c). While Flag-CHK1-CPs were easily detectable in basal state cells, the expression levels of endogenous CHK1-CPs were so low that only a few of their protein bands were detectable after prolonged exposure in our Western blot analysis. The pretreatment with the proteasome inhibitor MG132 increased multiple bands of CHK1-CPs to readily detectable levels (
Figure 5a), suggesting that the ubiquitin–proteasome pathway is involved in regulating the basal expression levels of CHK1-CPs. The current findings, which reveal an increase in ubiquitin modification of d270KD upon GA stimulation (
Figure 6b), emphasize the importance of identifying the ubiquitin ligase involved in this modification for a comprehensive understanding of the degradation mechanism of d270KD.
A previous study reported that Mule/HUWE1, a HECT-type ubiquitin E3 ligase, ubiquitinated CHK1 and played a critical role in regulating the basal level of CHK1 [
29]. In our HeLa cell model, we noted a decrease in endogenously and transiently expressed CHK1 turnover after the elimination of Mule, as shown in
Figure 7a,b. However, the knockdown of Mule did not affect the degradation of d270KD in response to the GA stimulation or suppress the formation of high-molecular-weight complexes, as shown in
Figure 7c. These results suggest that the degradation mechanism of d270KD, which is dependent on glycation modifications, involves a pathway distinct from the steady-state degradation mechanism of CHK1.
An important result from our Western blot analysis is that Mule was also affected by GA-induced glycation, as shown in
Figure 7c (Control siRNA). Specifically, the GA stimulation for 6 h resulted in a conspicuous smear of the putative monomer form of Mule. However, it remains unclear whether the decrease in single bands was a consequence of GA-AGE-modified Mule degradation induced by the GA stimulation or the formation of high-molecular-weight complexes. This uncertainty is attributed to the technical challenges associated with analyzing the behavior of GA-AGE-modified Mule over time because the transfer efficiency of high-molecular-weight proteins (more than 460 kDa) to a PVDF membrane is very low in a conventional Western blot analysis. The loss of function of ubiquitin ligases due to GA-AGE modifications may lead to the accumulation of proteins that need to be degraded, further exacerbating non-enzymatic glycation reactions and disrupting protein homeostasis. Therefore, a more comprehensive and detailed analysis of the effects of GA-induced glycation on ubiquitin ligases is warranted in the future.
Another possible cause of cellular damage due to GA-AGEs is proteopathy, a broad term that refers to the crosslinking of glycated proteins between molecules over time, resulting in the formation of larger high-molecular-weight complexes that gradually aggregate and accumulate, disrupting normal protein function [
3]. The proper folding of intracellular proteins is crucial for their biological functions, while aberrantly folded proteins may accumulate and aggregate in cells, leading to cellular stress. In response to this stress, cells activate mechanisms to monitor protein folding, such as the unfolded protein response in the endoplasmic reticulum [
30], which induces autophagy, an intracellular degradation mechanism that recycles damaged proteins and protects cells [
31,
32,
33,
34]. Non-enzymatic glycation modifications to intracellular proteins may lead to structural mutations and the formation of abnormal proteins, which may be recognized as aberrant by intracellular protein quality control mechanisms.
Extracellular AGEs have also been shown to activate intracellular autophagy pathways, which often have a cytoprotective role [
30,
31,
32,
33,
34]. Intracellularly formed AGEs were previously found to induce autophagy [
17]. For example, in a model in which cells were treated with MGO, a glycating substance, to accumulate intracellular AGEs (MG-H1), autophagy was induced through a pathway that involved p62. Importantly, while the loss of p62 promoted the accumulation of AGEs, p62 itself appeared to be affected by MGO-induced glycation over time, forming high-molecular-weight complexes and losing its function. In our experimental model of the formation of AGEs, in the RIPA-insoluble fraction, a slow, time-dependent increase in the expression level of a single band of p62 was observed in the Western blot analysis (
Figure 8a). In contrast, in the RIPA-soluble fraction, a gradual decrease was noted in the level of p62 starting 8 h after the GA stimulation. These results suggest that p62, which was present in the RIPA-soluble fraction, bound to the protein aggregates that gradually formed due to the GA stimulation and then migrated with them into the RIPA-insoluble fraction. In other words, the autophagy–lysosome pathway (and possibly the ubiquitin–proteasome degradation pathway) remains functional in the early stages of intracellular AGE formation, allowing for the proper clearance of AGEs. However, as glycation reactions continue, degradation pathways and other potential protective factors gradually transform into AGEs, resulting in the dysfunction of the AGE clearance pathway. This late stage of AGE formation may eventually induce apoptosis or passive, unprogrammed cell death by necrosis in affected cells [
3]. To the best of our knowledge, the induction of autophagy in the early stages of GA-AGE formation in cells has not yet been demonstrated.
We previously reported that the stimulation of a human pancreatic beta cell line (1.4E7 cells) with GA resulted in prominent cell death, accompanied by decreases in the autophagosome markers LC3-I and LC3-II, as well as p62 [
18]. These findings were observed after a prolonged stimulation (24 h) with 2 mM GA, at which stage, cell death was already induced. Therefore, important factors involved in various vital functions were modified by GA-AGEs, aggregated, and accumulated, suggesting that the GA-AGE degradation pathway was already disrupted in the late stage. To confirm the existence of an endogenous degradation and removal mechanism for GA-AGEs, a detailed analysis of the behavior of degradation-related factors from the initial stage of a glycation stimulation is needed.
In the present study, we investigated the behavior of p62, a known selective autophagy receptor, over 10 h, starting 2 h after stimulation with 2 mM GA. The results obtained from the RIPA-insoluble fraction revealed a slight increase in total endogenous p62 levels 10 h after the administration of GA (
Figure 8a). Since autophagy is responsible for the degradation of p62 when degrading its substrates, the accumulation of p62 is often observed when autophagy is impaired [
17]. However, total p62 levels remained unchanged in both the RIPA-soluble and insoluble fractions after 6 h of the stimulation with 2 mM GA; therefore, the complete failure of autophagy had not yet occurred 6 h after the GA stimulation. p62 plays a key role in substrate degradation by recruiting ubiquitinated cargo to autophagosomes [
35,
36]. The phosphorylation of the 403rd serine (Ser403) in the UBA domain of p62 by casein kinase-2 or TANK-binding kinase 1 increased its binding affinity to the ubiquitin chain, thereby facilitating selective autophagy [
37,
38].
Interestingly, after GA stimulation for 2 h, a gradual decrease in the phosphorylated form of p62 Ser403 was observed in the RIPA-soluble fraction, in contrast to an increase over time in the RIPA-insoluble fraction (
Figure 8a). Most of the d270KD high-molecular-weight complexes induced by the GA stimulation were present in the RIPA-insoluble fraction, and the level of d270KD high-molecular-weight complexes in this fraction peaked at approximately 6 h of GA stimulation, followed by a gradual decrease (
Figure 8a). Furthermore, the RIPA-insoluble fraction of the cells that did not express d270KD but were stimulated after 10 h of GA treatment and also showed an abundance of high-molecular-weight complexes that were positive for anti-TAGE antibodies (
Figure 8b). This result suggests that intracellular proteins modified with TAGEs (GA-AGEs) due to GA exposure form aggregates that are resistance to detergents, such as SDS, and are more likely to become insoluble intracellularly. Furthermore, the binding of phosphorylated p62 to ubiquitinated GA-AGEs may have been increased in the insoluble fraction, suggesting that the degradation system that uses selective autophagy was still partially functional during the initial stage of the GA stimulation, despite the formation of GA-AGE-modified aggregates. Importantly, immunoprecipitation experiments on the RIPA-insoluble fraction after the GA stimulation showed that the high-molecular-weight complexes of d270KD and p62 coprecipitated, and these complexes were also positive for anti-TAGE antibodies (
Figure 10). These results suggest that the high-molecular-weight complexes of d270KD in the RIPA-insoluble fraction were modified by GA-AGEs and also that p62 was one of its components.
Overall, the present results highlight the importance of analyzing the behavior of degradation-related factors, such as p62, from the initial stage of glycation stimulation to elucidate the endogenous GA-AGE degradation and removal mechanisms. Further research is needed to clarify the complex interplay between GA-AGE formation, autophagy, and cellular responses to proteopathy, which will provide a more detailed understanding of the pathogenesis of GA-AGE-related cellular damage. Since proteasome inhibitors completely suppressed the GA-stimulated rapid degradation of d270KD (
Figure 6a), the ubiquitin–proteasome pathway may also work in conjunction with the autophagy pathway during the early stages of GA-AGE formation to maintain intracellular protein homeostasis, which is disrupted by glycation modifications. The knockdown of p62 did not affect the degradation of d270KD induced by GA, but modestly reduced the formation of high-molecular-weight complexes derived from d270KD in the insoluble fraction (
Figure 9). Therefore, p62 may play a partial role in the formation of GA-AGE aggregates in the insoluble fraction.
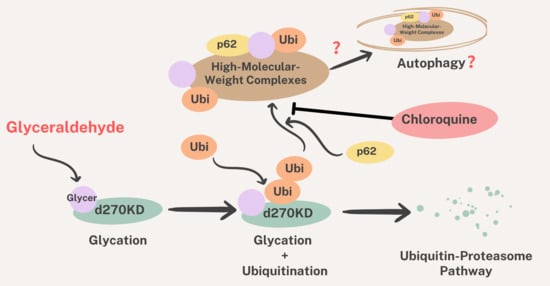
We also investigated the effects of chloroquine on autophagy. The results obtained revealed that the chloroquine treatment increased the basal level of p62 in the RIPA-insoluble fraction (
Figure 12b), indicating the potential inhibition of autophagy. However, the chloroquine treatment did not affect the GA-stimulated degradation of d270KD, but slightly suppressed the formation of high-molecular-weight complexes of d270KD in the RIPA-insoluble fraction, similar to the knockdown effect of p62 (
Figure 11 and
Figure 12). These results suggest that chloroquine interfered with the proper formation of high-molecular-weight complexes of d270KD, which is consistent with the knockdown effect of p62. One hypothesis is that p62 may be involved in sequestering randomly formed GA-AGEs throughout the cell by aggregating them, thereby preventing interference with the function of normal proteins and potentially protecting the cell. A more detailed analysis is needed on the subcellular localization of ubiquitinated and insoluble GA-AGE aggregates. However, it is important to note that the complete inhibition of autophagy by chloroquine was not fully confirmed in the present study. Therefore, the exact biological significance of the involvement of p62 in the formation of high-molecular-weight complexes of d270KD induced by GA stimulation remains to be elucidated. Further investigations are warranted to obtain a more detailed understanding of the autophagy-related effects of chloroquine in our experimental context.