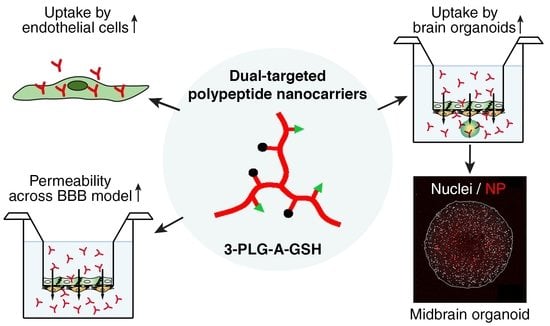
Targeting Human Endothelial Cells with Glutathione and Alanine Increases the Crossing of a Polypeptide Nanocarrier through a Blood–Brain Barrier Model and Entry to Human Brain Organoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Polypeptide Nanocarriers
2.3. Cell Cultures
2.4. Measurement of Cellular Viability
2.4.1. Impedance Measurements
2.4.2. Colorimetric Cytotoxicity Tests
2.5. Cellular Uptake of Polypeptide Nanocarriers, Visualization and Mechanisms of Internalization
2.6. Penetration of Nanocarriers across the Co-Culture Model of Blood–Brain Barrier
2.7. Permeability of Nanocarriers across the Blood–Brain Barrier and Internalization into Midbrain Organoids
2.8. Statistics
3. Results
3.1. Characterization of the Polypeptide Nanocarriers
3.2. Effect of Polypeptide Nanocarriers on the Viability of Brain Endothelial Cells
3.3. Cellular Uptake of Polypeptide Nanocarriers, Visualization and Mechanisms of Internalization
3.4. Penetration of Nanocarriers across the Co-Culture Model of Blood–Brain Barrier
3.5. Entry of Nanocarriers into Midbrain Organoids after Crossing the Blood–Brain Barrier
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tosi, G.; Duskey, J.T.; Kreuter, J. Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opin. Drug Deliv. 2020, 17, 23–32. [Google Scholar] [CrossRef]
- Abbott, N.J. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert. Opin. Drug. Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 10, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Günday Türeli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Masserini, M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013, 21, 238428. [Google Scholar] [CrossRef]
- Duro-Castano, A.; Moreira Leite, D.; Forth, J.; Deng, Y.; Matias, D.; Noble Jesus, C.; Battaglia, G. Designing peptide nanoparticles for efficient brain delivery. Adv. Drug Deliv. Rev. 2020, 160, 52–77. [Google Scholar]
- Zagorodko, O.; Arroyo-Crespo, J.J.; Nebot, V.J.; Vicent, M.J. Polypeptide-Based Conjugates as Therapeutics: Opportunities and Challenges. Macromol. Biosci. 2017, 17, 1. [Google Scholar] [CrossRef]
- Pham, T.N.; Su, C.F.; Huang, C.C.; Jan, J.S. Biomimetic hydrogels based on L-Dopa conjugated gelatin as pH-responsive drug carriers and antimicrobial agents. Colloids Surf. B. Biointerfaces 2020, 196, 111316. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chang, C.H.; Lin, C.Y.; Lin, L.F.; Yeh, M.L.; Jan, J.S. Disulfide-cross-linked PEG-block-polypeptide nanoparticles with high drug loading content as glutathione-triggered anticancer drug nanocarriers. Colloids Surf. B. Biointerfaces. 2018, 165, 172–181. [Google Scholar] [CrossRef]
- Johnson, L.C.; Akinmola, A.T.; Scholz, C. Poly(glutamic acid): From natto to drug delivery systems. Biocatal. Agric. Biotechnol. 2022, 40, 102292. [Google Scholar] [CrossRef]
- Su, C.F.; Chen, Y.F.; Tsai, Y.J.; Weng, S.M.; Jan, J.S. Antioxidant activity of linear and star-shaped polypeptides modified with dopamine and glutathione. Eur. Polym. J. 2021, 152, 110497. [Google Scholar] [CrossRef]
- Salmasi, Z.; Mokhtarzadeh, A.; Hashemi, M.; Ebrahimian, M.; Farzad, S.A.; Parhiz, H.; Ramezani, M. Effective and safe in vivo gene delivery based on polyglutamic acid complexes with heterocyclic amine modified-polyethylenimine. Colloids Surf. B. Biointerfaces. 2018, 172, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood-brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef]
- Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv. Drug. Deliv. Rev. 2014, 71, 2–14. [Google Scholar] [CrossRef]
- Walter, F.R.; Santa-Maria, A.R.; Mészáros, M.; Veszelka, S.; Dér, A.; Deli, M.A. Surface charge, glycocalyx, and blood-brain barrier function. Tissue Barriers 2021, 9, 1904773. [Google Scholar] [CrossRef]
- Sánchez-Navarro, M.; Teixidó, M.; Giralt, E. Jumping Hurdles: Peptides Able to Overcome Biological Barriers. Acc. Chem. Res. 2017, 50, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Vanlandewijck, M.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci. Data 2018, 21, 180160. [Google Scholar] [CrossRef]
- Vanlandewijck, M.; He, L.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef]
- Database of Gene Expression in Adult Mouse Brain and Lung Vascular and Perivascular Cells. Available online: https://betsholtzlab.org/VascularSingleCells/database.html (accessed on 15 September 2022).
- Veszelka, S.; Tóth, A.; Walter, F.R.; Tóth, A.E.; Gróf, I.; Mészáros, M.; Bocsik, A.; Hellinger, É.; Vastag, M.; Rákhely, G.; et al. Comparison of a rat primary cell-based blood-brain barrier model with epithelial and brain endothelial cell lines: Gene expression and drug transport. Front. Mol. Neurosci. 2018, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Campos-Bedolla, P.; Walter, F.R.; Veszelka, S.; Deli, M.A. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch. Med. Res. 2014, 45, 610–638. [Google Scholar]
- Mc Carthy, D.J.; Malhotra, M.; O’Mahony, A.M.; Cryan, J.F.; O’Driscoll, C.M. Nanoparticles and the blood-brain barrier: Advancing from in-vitro models towards therapeutic significance. Pharm. Res. 2015, 32, 1161–1185. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Hekmatara, T.; Dreis, S.; Vogel, T.; Gelperina, S.; Langer, K. Covalent attachment of apolipoprotein A-I and apolipoprotein B-100 to albumin nanoparticles enables drug transport into the brain. J. Control. Release 2007, 118, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Dal Magro, R.; Albertini, B.; Beretta, S.; Rigolio, R.; Donzelli, E.; Chiorazzi, A.; Ricci, M.; Blasi, P.; Sancini, G. Artificial apolipoprotein corona enables nanoparticle brain targeting. Nanomedicine 2018, 14, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Topal, G.R.; Mészáros, M.; Porkoláb, G.; Szecskó, A.; Polgár, T.F.; Siklós, L.; Deli, M.A.; Veszelka, S.; Bozkir, A. ApoE-Targeting Increases the Transfer of Solid Lipid Nanoparticles with Donepezil Cargo across a Culture Model of the Blood-Brain Barrier. Pharmaceutics. 2020, 29, 38. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar]
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the insulin receptor: Nanoparticles for drug delivery across the blood-brain barrier (BBB). J. Drug Target 2011, 19, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Mittur, A.; Bao, Y.; Tsuruo, T.; Kaplowitz, N. GSH transport in immortalized mouse brain endothelial cells: Evidence for apical localization of a sodium-dependent GSH transporter. J. Neurochem. 1999, 73, 390–399. [Google Scholar] [CrossRef]
- Gaillard, P.J. BBB crossing assessment and BBB crossing technologies in CNS Drug Discovery. Drug Discov. Today Technol. 2016, 20, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Birngruber, T.; Raml, R.; Gladdines, W.; Gatschelhofer, C.; Gander, E.; Ghosh, A.; Kroath, T.; Gaillard, P.J.; Pieber, T.R.; Sinner, F. Enhanced doxorubicin delivery to the brain administered through glutathione PEGylated liposomal doxorubicin (2B3-101) as compared with generic Caelyx,(®)/Doxil(®)-a cerebral open flow microperfusion pilot study. J. Pharm. Sci. 2014, 103, 1945–1948. [Google Scholar] [CrossRef]
- Kanhai, K.M.S.; Zuiker, R.G.J.A.; Stavrakaki, I.; Gladdines, W.; Gaillard, P.J.; Klaassen, E.S.; Groeneveld, G.J. Glutathione-PEGylated liposomal methylprednisolone in comparison to free methylprednisolone: Slow release characteristics and prolonged lymphocyte depression in a first-in-human study. Br. J. Clin. Pharmacol. 2018, 84, 1020–1028. [Google Scholar] [CrossRef]
- Veszelka, S.; Mészáros, M.; Kiss, L.; Kóta, Z.; Páli, T.; Hoyk, Z.; Bozsó, Z.; Fülöp, L.; Tóth, A.; Rákhely, G.; et al. Biotin and Glutathione Targeting of Solid Nanoparticles to Cross Human Brain Endothelial Cells. Curr. Pharm. Des. 2017, 23, 4198–4205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fekete, T.; Mészáros, M.; Szegletes, Z.; Vizsnyiczai, G.; Zimányi, L.; Deli, M.A.; Veszelka, S.; Kelemen, L. Optically Manipulated Microtools to Measure Adhesion of the Nanoparticle-Targeting Ligand Glutathione to Brain Endothelial Cells. ACS Appl. Mater. Interfaces 2021, 13, 39018–39029. [Google Scholar] [CrossRef]
- Dufes, C.; Gaillard, F.; Uchegbu, I.F.; Schätzlein, A.G.; Olivier, J.C.; Muller, J.M. Glucose-targeted niosomes deliver vasoactive intestinal peptide (VIP) to the brain. Int. J. Pharm. 2004, 285, 77–85. [Google Scholar] [CrossRef]
- Bragagni, M.; Mennini, N.; Ghelardini, C.; Mura, P. Development and characterization of niosomal formulations of doxorubicin aimed at brain targeting. J. Pharm. Pharm. Sci. 2012, 15, 184–196. [Google Scholar] [CrossRef]
- Woods, S.; O’Brien, L.M.; Butcher, W.; Preston, J.E.; Georgian, A.R.; Williamson, E.D.; Salguero, F.J.; Modino, F.; Abbott, N.J.; Roberts, C.W.; et al. Glucosamine-NISV delivers antibody across the blood-brain barrier: Optimization for treatment of encephalitic viruses. J. Control. Release 2020, 324, 644–656. [Google Scholar] [CrossRef]
- Gromnicova, R.; Davies, H.A.; Sreekanthreddy, P.; Romero, I.A.; Lund, T.; Roitt, I.M.; Phillips, J.B.; Male, D.K. Glucose-coated gold nanoparticles transfer across human brain endothelium and enter astrocytes in vitro. PLoS ONE 2013, 8, e81043. [Google Scholar] [CrossRef] [PubMed]
- Gromnicova, R.; Kaya, M.; Romero, I.A.; Williams, P.; Satchell, S.; Sharrack, B.; Male, D. Transport of gold nanoparticles by vascular endothelium from different human tissues. PLoS ONE 2016, 11, e0161610. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Gromnicova, R.; Loughlin, J.; Sharrack, B.; Male, D. Gold nanocarriers for transport of oligonucleotides across brain endothelial cells. PLoS ONE 2020, 15, e0236611. [Google Scholar] [CrossRef]
- Gonzalez-Carter, D.A.; Ong, Z.Y.; McGilvery, C.M.; Dunlop, I.E.; Dexter, D.T.; Porter, A.E. L-DOPA functionalized, multi-branched gold nanoparticles as brain-targeted nano-vehicles. Nanomedicine 2019, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, M.; Porkoláb, G.; Kiss, L.; Pilbat, A.M.; Kóta, Z.; Kupihár, Z.; Kéri, A.; Galbács, G.; Siklós, L.; Tóth, A.; et al. Niosomes decorated with dual ligands targeting brain endothelial transporters increase cargo penetration across the blood-brain barrier. Eur. J. Pharm. Sci. 2018, 123, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Porkoláb, G.; Mészáros, M.; Tóth, A.; Szecskó, A.; Harazin, A.; Szegletes, Z.; Ferenc, G.; Blastyák, A.; Mátés, L.; Rákhely, G.; et al. Combination of Alanine and Glutathione as Targeting Ligands of Nanoparticles Enhances Cargo Delivery into the Cells of the Neurovascular Unit. Pharmaceutics 2020, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Y.; Tang, C.C.; Jan, J.S. Synthesis and hydrogelation of star-shaped poly(l-lysine) polypeptides modified with different functional groups. Polymer 2018, 51, 108–116. [Google Scholar] [CrossRef]
- Huang, C.C.; Phan, T.H.M.; Ooya, T.; Kawasaki, S.; Lin, B.-Y.; Jan, J.-S. Effect of Tethered Sheet-like Motif and Asymmetric Topology on Hydrogelation of Star-Shaped Block Copolypeptides. Polymer 2022, 250, 124864. [Google Scholar] [CrossRef]
- Tang, C.C.; Zhang, S.H.; My Phan, T.H.; Tseng, Y.-C.; Jan, J.S. Block Length and To-pology Affect Self-Assembly and Gelation of Poly(L-lysine)-block- Poly(S-benzyl-L-cysteine) Block Copolypeptides. Polymer 2021, 228, 123891. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Li, X.; Zhao, D.; Sun, J.; Liu, X. Self-assembly and emulsification of dopamine-modified hyaluronan. Carbohydr. Polym. 2015, 123, 72–79. [Google Scholar] [CrossRef]
- Cecchelli, R.; Aday, S.; Sevin, E.; Almeida, C.; Culot, M.; Dehouck, L.; Coisne, C.; Engelhardt, B.; Dehouck, M.P.; Ferreira, L. A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PLoS ONE 2014, 17, e99733. [Google Scholar] [CrossRef]
- Mossu, A.; Rosito, M.; Khire, T.; Li Chung, H.; Nishihara, H.; Gruber, I.; Luke, E.; Dehouck, L.; Sallusto, F.; Gosselet, F.; et al. A silicon nanomembrane platform for the visualization of immune cell trafficking across the human blood-brain barrier under flow. J Cereb. Blood Flow Metab. 2019, 39, 395–410. [Google Scholar] [CrossRef]
- Nickels, S.L.; Modamio, J.; Mendes-Pinheiro, B.; Monzel, A.S.; Betsou, F.; Schwamborn, J.C. Reproducible generation of humanmidbrain organoids for in vitro modeling of Parkinson’s disease. Stem Cell Res. 2020, 46, 101870. [Google Scholar] [CrossRef]
- Veszelka, S.; Mészáros, M.; Porkoláb, G.; Szecskó, A.; Kondor, N.; Ferenc, G.; Polgár, T.F.; Katona, G.; Kóta, Z.; Kelemen, L.; et al. A Triple Combination of Targeting Ligands Increases the Penetration of Nanoparticles across a Blood-Brain Barrier Culture Model. Pharmaceutics 2022, 14, 86. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Moya, E.L.J.; Lombardo, S.M.; Vandenhaute, E.; Schneider, M.; Mysiorek, C.; Türeli, A.E.; Kanda, T.; Shimizu, F.; Sano, Y.; Maubon, N.; et al. Interaction of surfactant coated PLGA nanoparticles with in vitro human brain-like endothelial cells. Int. J. Pharm. 2022, 10, 121780. [Google Scholar] [CrossRef]
- Oldham, E.A.; Li, C.; Ke, S.; Wallace, S.; Huang, P. Comparison of action of paclitaxel and poly(L-glutamic acid)-paclitaxel conjugate in human breast cancer cells. Int. J. Oncol. 2000, 16, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Niño-Pariente, A.; Armiñán, A.; Reinhard, S.; Scholz, C.; Kos, P.; Wagner, E.; Vicent, M.J. Design of Poly-l-Glutamate-Based Complexes for pDNA Delivery. Macromol. Biosci. 2017, 17, 10. [Google Scholar] [CrossRef]
- Duro-Castano, A.; Nebot, V.J.; Niño-Pariente, A.; Armiñán, A.; Arroyo-Crespo, J.J.; Paul, A.; Feiner-Gracia, N.; Albertazzi, L.; Vicent, M.J. Capturing "Extraordinary" Soft-Assembled Charge-Like Polypeptides as a Strategy for Nanocarrier Design. Adv. Mater. 2017, 29, 39. [Google Scholar] [CrossRef] [PubMed]
- Mamdouh, Z.; Giocondi, M.C.; Laprade, R.; Le Grimellec, C. Temperature dependence of endocytosis in renal epithelial cells in culture. Biochim. Biophys. Acta. 1996, 1282, 171–173. [Google Scholar] [CrossRef]
- Maussang, D.; Rip, J.; van Kregten, J.; van den Heuvel, A.; van der Pol, S.; van der Boom, B.; Reijerkerk, A.; Chen, L.; de Boer, M.; Gaillard, P.; et al. Glutathione conjugation dose-dependently increases brain-specific liposomal drug delivery in vitro and in vivo. Drug Discov. Today Technol. 2016, 20, 59–69. [Google Scholar] [CrossRef]
- Santa-Maria, A.R.; Walter, F.R.; Figueiredo, R.; Kincses, A.; Vigh, J.P.; Heymans, M.; Culot, M.; Winter, P.; Gosselet, F.; Dér, A.; et al. Flow induces barrier and glycocalyx-related genes and negative surface charge in a lab-on-a-chip human blood-brain barrier model. J. Cereb. Blood Flow Metab. 2021, 41, 2201–2215. [Google Scholar] [CrossRef]
- Nelemans, L.C.; Gurevich, L. Drug Delivery with Polymeric Nanocarriers-Cellular Uptake Mechanisms. Materials 2020, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.E.; Holst, M.R.; Nielsen, M.S. Vesicular Transport Machinery in Brain Endothelial Cells: What We Know and What We Do not. Curr. Pharm. Des. 2020, 26, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Bak, M.; Kempen, P.J.; Melander, F.; Burkhart, A.; Thomsen, M.S.; Nielsen, M.S.; Moos, T.; Andresen, T.L. Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles. Theranostics. 2018, 8, 3416–3436. [Google Scholar] [CrossRef]
- Leite, P.E.C.; Pereira, M.R.; Harris, G.; Pamies, D.; Dos Santos, L.M.G.; Granjeiro, J.M.; Hogberg, H.T.; Hartung, T.; Smirnova, L. Suitability of 3D human brain spheroid models to distinguish toxic effects of gold and poly-lactic acid nanoparticles to assess biocompatibility for brain drug delivery. Part Fibre Toxicol. 2019, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Modamio, J.; Saraiva, C.; Giro, G.G.; Nickels, S.L.; Jarazo, J.; Antony, P.; Barbuti, P.; Hadler, R.; Jäger, C.; Krüger, R.; et al. Synaptic decline precedes dopaminergic neuronal loss in human midbrain organoids harboring a triplication of the SNCA gene. bioRxiv 2021, 452499. [Google Scholar] [CrossRef]
- Smits, L.M.; Schwamborn, J.C. Midbrain Organoids: A New Tool to Investigate Parkinson’s Disease. Front. Cell Dev. Biol. 2020, 8, 359. [Google Scholar] [CrossRef]










| Nanocarrier | Size (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|
| 3−PLG | 263.10 ± 37.90 | 0.39 ± 0.01 | −25.67 ± 1.57 |
| 3−PLG−A−GSH | 185.13 ± 07.59 | 0.39 ± 0.01 | −14.00 ± 0.82 |
| Nanocarriers | Previous Papers [43,44] | Present Manuscript |
|---|---|---|
| Type | niosome | polypeptide |
| Shape | nanovesicle, spherical | 3-armed, filamentous |
| Composition | non-ionic surfactants cholesterol | poly(L-glutamic acid γ-benzyl ester) |
| Ligands | dodecanoyl alanine DSPE-PEG-glutathione | l-alanine l-glutathione |
| Preparation | lipid film hydratation | ring opening polymerization |
| Size | 103 and 115 nm | 185 nm |
| Charge | −7 and −5 mV | −14 mV |
| Cargo | albumin (65 kDa) mCherry (27 kDa) | rhodamine 6G (0.5 kDa) |
| Fluorescent marker | Evans blue, mCherry | rhodamine 6G |
| Model | Previous Papers [43,44] | Present Manuscript |
|---|---|---|
| Species | rat | human |
| Endothelial cell type | primary | stem cell-derived |
| Co-culture with | brain pericytes and astrocytes | brain pericytes |
| Cellular uptake after BBB crossing | astrocytes [44] | human brain organoids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mészáros, M.; Phan, T.H.M.; Vigh, J.P.; Porkoláb, G.; Kocsis, A.; Páli, E.K.; Polgár, T.F.; Walter, F.R.; Bolognin, S.; Schwamborn, J.C.; et al. Targeting Human Endothelial Cells with Glutathione and Alanine Increases the Crossing of a Polypeptide Nanocarrier through a Blood–Brain Barrier Model and Entry to Human Brain Organoids. Cells 2023, 12, 503. https://doi.org/10.3390/cells12030503
Mészáros M, Phan THM, Vigh JP, Porkoláb G, Kocsis A, Páli EK, Polgár TF, Walter FR, Bolognin S, Schwamborn JC, et al. Targeting Human Endothelial Cells with Glutathione and Alanine Increases the Crossing of a Polypeptide Nanocarrier through a Blood–Brain Barrier Model and Entry to Human Brain Organoids. Cells. 2023; 12(3):503. https://doi.org/10.3390/cells12030503
Chicago/Turabian StyleMészáros, Mária, Thi Ha My Phan, Judit P. Vigh, Gergő Porkoláb, Anna Kocsis, Emese K. Páli, Tamás F. Polgár, Fruzsina R. Walter, Silvia Bolognin, Jens C. Schwamborn, and et al. 2023. "Targeting Human Endothelial Cells with Glutathione and Alanine Increases the Crossing of a Polypeptide Nanocarrier through a Blood–Brain Barrier Model and Entry to Human Brain Organoids" Cells 12, no. 3: 503. https://doi.org/10.3390/cells12030503
APA StyleMészáros, M., Phan, T. H. M., Vigh, J. P., Porkoláb, G., Kocsis, A., Páli, E. K., Polgár, T. F., Walter, F. R., Bolognin, S., Schwamborn, J. C., Jan, J. -S., Deli, M. A., & Veszelka, S. (2023). Targeting Human Endothelial Cells with Glutathione and Alanine Increases the Crossing of a Polypeptide Nanocarrier through a Blood–Brain Barrier Model and Entry to Human Brain Organoids. Cells, 12(3), 503. https://doi.org/10.3390/cells12030503