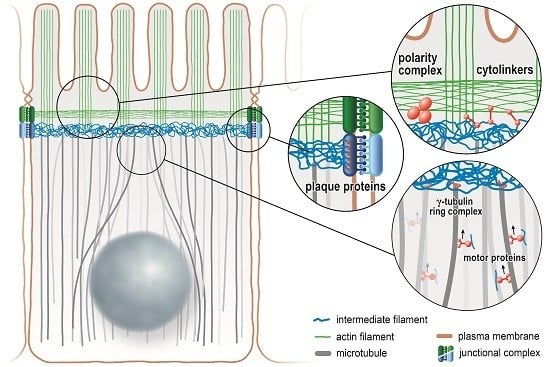
Intermediate Filaments and Polarization in the Intestinal Epithelium
Abstract
:1. Introduction
2. The Intermediate Filament Cytoskeleton of the Intestinal Epithelium Is Characterized by Cell Type-Specific Polypeptide Subunits
3. Intermediate Filaments Are Needed for the Integrity and Function of Intestinal Epithelial Cells
4. The Polarized Distribution of Intermediate Filaments in Intestinal Cells Is Evolutionarily Conserved
5. Intermediate Filaments Are Anchored to the Apical Junction Complex in the Intestinal Epithelium
6. Intermediate Filaments Are Linked to the Actin and Microtubule Cytoskeleton in Intestinal Cells
7. Intermediate Filaments Affect the Distribution of Membrane Proteins in Polarized Epithelia
8. Intermediate Filaments Interact with the PAR-aPKC Polarity Complex in Polarized Epithelia
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Straube, A. Microtubules and microtubule-associated proteins (MAPs). In Encyclopedia of Cell Biology, 1st ed.; Bradshaw, R.A., Stahl, P.D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 2, pp. 539–547. [Google Scholar]
- Heissler, S.M.; Sellers, J.R. Myosins. In Encyclopedia of Cell Biology, 1st ed.; Bradshaw, R.A., Stahl, P.D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 2, pp. 597–607. [Google Scholar]
- Pernier, J.; Montaville, P.; Carlier, M.F. Actin assembly dynamics and its regulation in motile and morphogenetic processes. In Encyclopedia of Cell Biology, 1st ed.; Bradshaw, R.A., Stahl, P.D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 2, pp. 548–568. [Google Scholar]
- Leube, R.E.; Schwarz, N. Intermediate filaments. In Encyclopedia of Cell Biology, 1st ed.; Bradshaw, R.A., Stahl, P.D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 2, pp. 569–578. [Google Scholar]
- Omary, M.B. “IF-pathies”: A broad spectrum of intermediate filament-associated diseases. J. Clin. Invest. 2009, 119, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Lane, E.B. Intermediate filaments and stress. Exp. Cell Res. 2007, 313, 2244–2254. [Google Scholar] [CrossRef] [PubMed]
- Toivola, D.M.; Strnad, P.; Habtezion, A.; Omary, M.B. Intermediate filaments take the heat as stress proteins. Trends Cell Biol. 2010, 20, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Geisler, F.; Leube, R.E. Epithelial intermediate filaments: Guardians against microbial infection? Cells 2016, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Jahnel, O.; Hoffmann, B.; Merkel, R.; Bossinger, O.; Leube, R.E. Mechanical probing of the intermediate filament-rich Caenorhabditis elegans intestine. Methods Enzymol. 2016, 568, 681–706. [Google Scholar] [PubMed]
- Schwarz, N.; Windoffer, R.; Magin, T.M.; Leube, R.E. Dissection of keratin network formation, turnover and reorganization in living murine embryos. Sci. Rep. 2015, 5, 9007. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.W.; Lane, E.B. The quest for the function of simple epithelial keratins. Bioessays 2003, 25, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Omary, M.B.; Ku, N.O.; Strnad, P.; Hanada, S. Toward unraveling the complexity of simple epithelial keratins in human disease. J. Clin. Invest. 2009, 119, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, D.; Tiernan, J.P.; Lobo, A.J.; Evans, C.A.; Corfe, B.M. Keratins in colorectal epithelial function and disease. Int. J. Exp. Pathol. 2012, 93, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.W.; Lane, E.B. Keratin mutations and intestinal pathology. J. Pathol. 2004, 204, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Toivola, D.M.; Feng, N.; Greenberg, H.B.; Franke, W.W.; Omary, M.B. Keratin 20 helps maintain intermediate filament organization in intestinal epithelia. Mol. Biol. Cell 2003, 14, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Schiller, D.L.; Franke, W.W. Identification of protein it of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns. J. Cell Biol. 1990, 111, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Zimbelmann, R.; Goldschmidt, M.D.; Keith, M.; Laufer, J.; Kasper, M.; Koch, P.J.; Franke, W.W. The human gene encoding cytokeratin 20 and its expression during fetal development and in gastrointestinal carcinomas. Differentiation 1993, 53, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Calnek, D.; Quaroni, A. Differential localization by in situ hybridization of distinct keratin mRNA species during intestinal epithelial cell development and differentiation. Differentiation 1993, 53, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Quaroni, A.; Calnek, D.; Quaroni, E.; Chandler, J.S. Keratin expression in rat intestinal crypt and villus cells. Analysis with a panel of monoclonal antibodies. J. Biol. Chem. 1991, 266, 11923–11931. [Google Scholar] [PubMed]
- Baribault, H.; Penner, J.; Iozzo, R.V.; Wilson-Heiner, M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994, 8, 2964–2973. [Google Scholar] [CrossRef] [PubMed]
- Ameen, N.A.; Figueroa, Y.; Salas, P.J. Anomalous apical plasma membrane phenotype in CK8-deficient mice indicates a novel role for intermediate filaments in the polarization of simple epithelia. J. Cell Sci. 2001, 114, 563–575. [Google Scholar] [PubMed]
- Asghar, M.N.; Silvander, J.S.; Helenius, T.O.; Lahdeniemi, I.A.; Alam, C.; Fortelius, L.E.; Holmsten, R.O.; Toivola, D.M. The amount of keratins matters for stress protection of the colonic epithelium. PLoS ONE 2015, 10, e0127436. [Google Scholar] [CrossRef] [PubMed]
- Birkenkamp-Demtroder, K.; Mansilla, F.; Sorensen, F.B.; Kruhoffer, M.; Cabezon, T.; Christensen, L.L.; Aaltonen, L.A.; Verspaget, H.W.; Orntoft, T.F. Phosphoprotein keratin 23 accumulates in MSS but not MSI colon cancers in vivo and impacts viability and proliferation in vitro. Mol. Oncol. 2007, 1, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Winter, H.; Langbein, L.; Bleiler, R.; Schweizer, J. The human type I keratin gene family: Characterization of new hair follicle specific members and evaluation of the chromosome 17q21.2 gene domain. Differentiation 2004, 72, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, E.; Itin, P.; Whittock, N.V.; McGrath, J.A.; Meyer, R.; DiGiovanna, J.J.; Bale, S.J.; Uitto, J.; Richard, G. Refined mapping of Naegeli-Franceschetti-Jadassohn syndrome to a 6 cM interval on chromosome 17q11.2-q21 and investigation of candidate genes. J. Invest. Dermatol. 2002, 119, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Strelkov, S.V.; Herrmann, H.; Aebi, U. Molecular architecture of intermediate filaments. Bioessays 2003, 25, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Hesse, M.; Reichenzeller, M.; Aebi, U.; Magin, T.M. Functional complexity of intermediate filament cytoskeletons: From structure to assembly to gene ablation. Int. Rev. Cytol. 2003, 223, 83–175. [Google Scholar] [PubMed]
- Hofmann, I.; Franke, W.W. Heterotypic interactions and filament assembly of type I and type II cytokeratins in vitro: Viscometry and determinations of relative affinities. Eur. J. Cell Biol. 1997, 72, 122–132. [Google Scholar] [PubMed]
- Iwatsuki, H.; Suda, M. Seven kinds of intermediate filament networks in the cytoplasm of polarized cells: Structure and function. Acta Histochem. Cytochem. 2010, 43, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B. Establishment of gut fate in the E lineage of C. elegans: The roles of lineage-dependent mechanisms and cell interactions. Development 1993, 118, 1267–1277. [Google Scholar] [PubMed]
- Leung, B.; Hermann, G.J.; Priess, J.R. Organogenesis of the Caenorhabditis elegans intestine. Dev. Biol. 1999, 216, 114–134. [Google Scholar] [CrossRef] [PubMed]
- McGhee, J.D. The C. elegans Intestine. Available online: http://www.wormbook.org/chapters/www_intestine/intestine.html (accessed on 15 July 2016).
- Dodemont, H.; Riemer, D.; Ledger, N.; Weber, K. Eight genes and alternative RNA processing pathways generate an unexpectedly large diversity of cytoplasmic intermediate filament proteins in the nematode Caenorhabditis elegans. EMBO J. 1994, 13, 2625–2638. [Google Scholar] [PubMed]
- Karabinos, A.; Schmidt, H.; Harborth, J.; Schnabel, R.; Weber, K. Essential roles for four cytoplasmic intermediate filament proteins in Caenorhabditis elegans development. Proc. Natl. Acad. Sci. USA 2001, 98, 7863–7868. [Google Scholar] [CrossRef] [PubMed]
- Carberry, K.; Wiesenfahrt, T.; Windoffer, R.; Bossinger, O.; Leube, R.E. Intermediate filaments in Caenorhabditis elegans. Cell Motil. Cytoskeleton 2009, 66, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Karabinos, A.; Schunemann, J.; Weber, K. Most genes encoding cytoplasmic intermediate filament (IF) proteins of the nematode Caenorhabditis elegans are required in late embryogenesis. Eur. J. Cell Biol. 2004, 83, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Hüsken, K.; Wiesenfahrt, T.; Abraham, C.; Windoffer, R.; Bossinger, O.; Leube, R.E. Maintenance of the intestinal tube in Caenorhabditis elegans: The role of the intermediate filament protein IFC-2. Differentiation 2008, 76, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Karabinos, A.; Schulze, E.; Schunemann, J.; Parry, D.A.; Weber, K. In vivo and in vitro evidence that the four essential intermediate filament (IF) proteins A1, A2, A3 and B1 of the nematode Caenorhabditis elegans form an obligate heteropolymeric IF system. J. Mol. Biol. 2003, 333, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Karabinos, A.; Schunemann, J.; Parry, D.A.; Weber, K. Tissue-specific co-expression and in vitro heteropolymer formation of the two small branchiostoma intermediate filament proteins A3 and B2. J. Mol. Biol. 2002, 316, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Karabinos, A.; Schulze, E.; Klisch, T.; Wang, J.; Weber, K. Expression profiles of the essential intermediate filament (IF) protein A2 and the IF protein C2 in the nematode Caenorhabditis elegans. Mech. Dev. 2002, 117, 311–314. [Google Scholar] [CrossRef]
- Bossinger, O.; Fukushige, T.; Claeys, M.; Borgonie, G.; McGhee, J.D. The apical disposition of the Caenorhabditis elegans intestinal terminal web is maintained by LET-413. Dev. Biol. 2004, 268, 448–456. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, A.J.; Baggett, J.J.; Perumov, N.; Bauer, R.A.; Januszewski, T.; Schriefer, L.; Waddle, J.A. ACT-5 is an essential Caenorhabditis elegans actin required for intestinal microvilli formation. Mol. Biol. Cell 2005, 16, 3247–3259. [Google Scholar] [CrossRef] [PubMed]
- Segbert, C.; Johnson, K.; Theres, C.; van Furden, D.; Bossinger, O. Molecular and functional analysis of apical junction formation in the gut epithelium of Caenorhabditis elegans. Dev. Biol. 2004, 266, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Bouameur, J.E.; Bar, J.; Rice, R.H.; Hornig-Do, H.T.; Roop, D.R.; Schwarz, N.; Brodesser, S.; Thiering, S.; Leube, R.E.; et al. A keratin scaffold regulates epidermal barrier formation, mitochondrial lipid composition, and activity. J. Cell Biol. 2015, 211, 1057–1075. [Google Scholar] [CrossRef] [PubMed]
- Tamai, Y.; Ishikawa, T.; Bosl, M.R.; Mori, M.; Nozaki, M.; Baribault, H.; Oshima, R.G.; Taketo, M.M. Cytokeratins 8 and 19 in the mouse placental development. J. Cell Biol. 2000, 151, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Magin, T.M.; Schroder, R.; Leitgeb, S.; Wanninger, F.; Zatloukal, K.; Grund, C.; Melton, D.W. Lessons from keratin 18 knockout mice: Formation of novel keratin filaments, secondary loss of keratin 7 and accumulation of liver-specific keratin 8-positive aggregates. J. Cell Biol. 1998, 140, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M.; Grund, C.; Herrmann, H.; Brohl, D.; Franz, T.; Omary, M.B.; Magin, T.M. A mutation of keratin 18 within the coil 1A consensus motif causes widespread keratin aggregation but cell type-restricted lethality in mice. Exp. Cell Res. 2007, 313, 3127–3140. [Google Scholar] [CrossRef] [PubMed]
- Ku, N.O.; Michie, S.; Oshima, R.G.; Omary, M.B. Chronic hepatitis, hepatocyte fragility, and increased soluble phosphoglycokeratins in transgenic mice expressing a keratin 18 conserved arginine mutant. J. Cell Biol. 1995, 131, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Toivola, D.M.; Krishnan, S.; Binder, H.J.; Singh, S.K.; Omary, M.B. Keratins modulate colonocyte electrolyte transport via protein mistargeting. J. Cell Biol. 2004, 164, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A.; Toivola, D.M.; Butcher, E.C.; Omary, M.B. Keratin-8-deficient mice develop chronic spontaneous th2 colitis amenable to antibiotic treatment. J. Cell Sci. 2005, 118, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A.; Toivola, D.M.; Asghar, M.N.; Kronmal, G.S.; Brooks, J.D.; Butcher, E.C.; Omary, M.B. Absence of keratin 8 confers a paradoxical microflora-dependent resistance to apoptosis in the colon. Proc. Natl. Acad. Sci. USA 2011, 108, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; Smith, F.J.; Lunny, D.P.; Campbell, L.E.; Davidson, K.M.; MacCallum, S.F.; Corden, L.D.; Christie, L.; Fleming, S.; Lane, E.B.; et al. Generation and characterisation of keratin 7 (K7) knockout mice. PLoS ONE 2013, 8, e64404. [Google Scholar] [CrossRef] [PubMed]
- Baribault, H.; Price, J.; Miyai, K.; Oshima, R.G. Mid-gestational lethality in mice lacking keratin 8. Genes Dev. 1993, 7, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.U.; Baumgart, D.C.; Sturm, A. Review article: The aetiopathogenesis of inflammatory bowel disease—Immunology and repair mechanisms. Aliment. Pharmacol. Ther. 2004, 20 (Suppl. S4), 9–17. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Turner, J.R. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: Tight junction dynamics exposed. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G577–G582. [Google Scholar] [CrossRef] [PubMed]
- Zupancic, T.; Stojan, J.; Lane, E.B.; Komel, R.; Bedina-Zavec, A.; Liovic, M. Intestinal cell barrier function in vitro is severely compromised by keratin 8 and 18 mutations identified in patients with inflammatory bowel disease. PLoS ONE 2014, 9, e99398. [Google Scholar]
- Wang, L.; Srinivasan, S.; Theiss, A.L.; Merlin, D.; Sitaraman, S.V. Interleukin-6 induces keratin expression in intestinal epithelial cells: Potential role of keratin-8 in interleukin-6-induced barrier function alterations. J. Biol. Chem. 2007, 282, 8219–8227. [Google Scholar] [CrossRef] [PubMed]
- Carberry, K.; Wiesenfahrt, T.; Geisler, F.; Stocker, S.; Gerhardus, H.; Uberbach, D.; Davis, W.; Jorgensen, E.; Leube, R.E.; Bossinger, O. The novel intestinal filament organizer IFO-1 contributes to epithelial integrity in concert with ERM-1 and DLG-1. Development 2012, 139, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Boulan, E.; Nelson, W.J. Morphogenesis of the polarized epithelial cell phenotype. Science 1989, 245, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.M.; Mostov, K.E. From cells to organs: Building polarized tissue. Nat. Rev. Mol. Cell Biol. 2008, 9, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Tilney, L.G.; Fujiwara, K.; Heuser, J.E. Organization of actin, myosin, and intermediate filaments in the brush border of intestinal epithelial cells. J. Cell Biol. 1982, 94, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Bement, W.M.; Mooseker, M.S. The cytoskeleton of the intestinal epithelium: Components, assembly, and dynamic rearrangements. In The Cytoskeleton: A Multi-Volume Treatise; John, E.H., Ian, F.P., Eds.; JAI: Stamford, CT, USA, 1996; Volume 3, pp. 359–404. [Google Scholar]
- Drenckhahn, D.; Groschel-Stewart, U. Localization of myosin, actin, and tropomyosin in rat intestinal epithelium: Immunohistochemical studies at the light and electron microscope levels. J. Cell Biol. 1980, 86, 475–482. [Google Scholar] [PubMed]
- Drenckhahn, D.; Dermietzel, R. Organization of the actin filament cytoskeleton in the intestinal brush border: A quantitative and qualitative immunoelectron microscope study. J. Cell Biol. 1988, 107, 1037–1048. [Google Scholar] [PubMed]
- Menard, S.; Cerf-Bensussan, N.; Heyman, M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 2010, 3, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Franke, W.W.; Appelhans, B.; Schmid, E.; Freudenstein, C.; Osborn, M.; Weber, K. The organization of cytokeratin filaments in the intestinal epithelium. Eur. J. Cell Biol. 1979, 19, 255–268. [Google Scholar] [PubMed]
- Hemmi, A.; Komiyama, A.; Ohno, S.; Fujii, Y.; Kawaoi, A.; Katoh, R.; Suzuki, K. Different organization of intermediate filaments in columnar cells of rat large intestinal mucosa as revealed by confocal laser scanning microscopy and quick-freezing and deep-etching method. Virchows Arch. 1995, 426, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.T.; Anderton, B.H.; Wylie, C.C. The organization of intermediate filaments in normal human colonic epithelium and colonic carcinoma cells. Int. J. Cancer 1983, 32, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Brunser, O.; Luft, H.J. Fine structure of the apex of absorptive cell from rat small intestine. J. Ultrastruct. Res. 1970, 31, 291–311. [Google Scholar] [CrossRef]
- Gallicano, G.I.; Kouklis, P.; Bauer, C.; Yin, M.; Vasioukhin, V.; Degenstein, L.; Fuchs, E. Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J. Cell Biol. 1998, 143, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Kröger, C.; Loschke, F.; Schwarz, N.; Windoffer, R.; Leube, R.E.; Magin, T.M. Keratins control intercellular adhesion involving PKC-α-mediated desmoplakin phosphorylation. J. Cell Biol. 2013, 201, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Holthöfer, B.; Windoffer, R.; Troyanovsky, S.; Leube, R.E. Structure and function of desmosomes. Int. Rev. Cytol. 2007, 264, 65–163. [Google Scholar] [PubMed]
- Getsios, S.; Huen, A.C.; Green, K.J. Working out the strength and flexibility of desmosomes. Nat. Rev. Mol. Cell Biol. 2004, 5, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, C.; Ciolfi, S.; Caroti, D.; Lupetti, P.; Dallai, R. Isomin: A novel cytoplasmic intermediate filament protein from an arthropod species. BMC Biol. 2011, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Munn, E.A.; Greenwood, C.A. The occurrence of submicrovillar endotube (modified terminal web) and associated structures in the intestinal epithelia of nematodes. Philos. Trans. R. Soc. Lond. B 1984, 306, 1–18. [Google Scholar] [CrossRef]
- Green, K.J.; Getsios, S.; Troyanovsky, S.; Godsel, L.M. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb. Perspect. Biol. 2010, 2, a000125. [Google Scholar] [CrossRef] [PubMed]
- Sumigray, K.D.; Lechler, T. Desmoplakin controls microvilli length but not cell adhesion or keratin organization in the intestinal epithelium. Mol. Biol. Cell 2012, 23, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Knust, E.; Bossinger, O. Composition and formation of intercellular junctions in epithelial cells. Science 2002, 298, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Pasti, G.; Labouesse, M. Epithelial Junctions, Cytoskeleton, and Polarity. Available online: http://www.wormbook.org/chapters/www_epithelialjunctionsattach.2/epithelialjunctions.html (accessed on 15 July 2016).
- Asano, A.; Asano, K.; Sasaki, H.; Furuse, M.; Tsukita, S. Claudins in Caenorhabditis elegans: Their distribution and barrier function in the epithelium. Curr. Biol. 2003, 13, 1042–1046. [Google Scholar] [CrossRef]
- Costa, M.; Raich, W.; Agbunag, C.; Leung, B.; Hardin, J.; Priess, J.R. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J. Cell Biol. 1998, 141, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Köppen, M.; Simske, J.S.; Sims, P.A.; Firestein, B.L.; Hall, D.H.; Radice, A.D.; Rongo, C.; Hardin, J.D. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat. Cell Biol. 2001, 3, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Bossinger, O.; Klebes, A.; Segbert, C.; Theres, C.; Knust, E. Zonula adherens formation in Caenorhabditis elegans requires DLG-1, the homologue of the drosophila gene discs large. Dev. Biol. 2001, 230, 29–42. [Google Scholar] [CrossRef] [PubMed]
- McMahon, L.; Legouis, R.; Vonesch, J.L.; Labouesse, M. Assembly of C. elegans apical junctions involves positioning and compaction by LET-413 and protein aggregation by the MAGUK protein DLG-1. J. Cell Sci. 2001, 114, 2265–2277. [Google Scholar] [PubMed]
- Legouis, R.; Gansmuller, A.; Sookhareea, S.; Bosher, J.M.; Baillie, D.L.; Labouesse, M. LET-413 is a basolateral protein required for the assembly of adherens junctions in Caenorhabditis elegans. Nat. Cell Biol. 2000, 2, 415–422. [Google Scholar] [PubMed]
- Grimm-Günter, E.M.; Revenu, C.; Ramos, S.; Hurbain, I.; Smyth, N.; Ferrary, E.; Louvard, D.; Robine, S.; Rivero, F. Plastin 1 binds to keratin and is required for terminal web assembly in the intestinal epithelium. Mol. Biol. Cell 2009, 20, 2549–2562. [Google Scholar] [CrossRef] [PubMed]
- Salas, P.J.; Rodriguez, M.L.; Viciana, A.L.; Vega-Salas, D.E.; Hauri, H.P. The apical submembrane cytoskeleton participates in the organization of the apical pole in epithelial cells. J. Cell Biol. 1997, 137, 359–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosher, J.M.; Hahn, B.S.; Legouis, R.; Sookhareea, S.; Weimer, R.M.; Gansmuller, A.; Chisholm, A.D.; Rose, A.M.; Bessereau, J.L.; Labouesse, M. The Caenorhabditis elegans VAB-10 spectraplakin isoforms protect the epidermis against internal and external forces. J. Cell Biol. 2003, 161, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Fievet, B.; Louvard, D.; Arpin, M. ERM proteins in epithelial cell organization and functions. Biochim. Biophys. Acta 2007, 1773, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Fehon, R.G.; McClatchey, A.I.; Bretscher, A. Organizing the cell cortex: The role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010, 11, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Salas, P.J. Insoluble γ-tubulin-containing structures are anchored to the apical network of intermediate filaments in polarized CACO-2 epithelial cells. J. Cell Biol. 1999, 146, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Oriolo, A.S.; Wald, F.A.; Canessa, G.; Salas, P.J. GCP6 binds to intermediate filaments: A novel function of keratins in the organization of microtubules in epithelial cells. Mol. Biol. Cell 2007, 18, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Sumigray, K.D.; Chen, H.; Lechler, T. Lis1 is essential for cortical microtubule organization and desmosome stability in the epidermis. J. Cell Biol. 2011, 194, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Sumigray, K.D.; Lechler, T. Control of cortical microtubule organization and desmosome stability by centrosomal proteins. Bioarchitecture 2011, 1, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Lechler, T.; Fuchs, E. Desmoplakin: An unexpected regulator of microtubule organization in the epidermis. J. Cell Biol. 2007, 176, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Wacker, I.U.; Rickard, J.E.; de Mey, J.R.; Kreis, T.E. Accumulation of a microtubule-binding protein, pp170, at desmosomal plaques. J. Cell Biol. 1992, 117, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Shu, T.; Sanada, K.; Lariviere, R.C.; Tseng, H.C.; Park, S.K.; Julien, J.P.; Tsai, L.H. A NUDEL-dependent mechanism of neurofilament assembly regulates the integrity of CNS neurons. Nat. Cell Biol. 2004, 6, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Bobinnec, Y.; Fukuda, M.; Nishida, E. Identification and characterization of Caenorhabditis elegans γ-tubulin in dividing cells and differentiated tissues. J. Cell Sci. 2000, 113, 3747–3759. [Google Scholar] [PubMed]
- Quintin, S.; Gally, C.; Labouesse, M. Noncentrosomal microtubules in C. elegans epithelia. Genesis 2016, 54, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, D.; Quintin, S.; Green, R.A.; Cheerambathur, D.K.; Ochoa, S.D.; Desai, A.; Oegema, K. NOCA-1 functions with γ-tubulin and in parallel to patronin to assemble non-centrosomal microtubule arrays in C. elegans. Elife 2015, 4, e08649. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj, P.; Kroger, C.; Reuter, U.; Windoffer, R.; Leube, R.E.; Magin, T.M. Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and raptor. J. Cell Biol. 2009, 187, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Inoko, A.; Shiromizu, T.; Nakayama, M.; Zou, P.; Yonemura, S.; Hayashi, Y.; Izawa, I.; Sasoh, M.; Uji, Y.; et al. The keratin-binding protein albatross regulates polarization of epithelial cells. J. Cell Biol. 2008, 183, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Ohno, S. The PAR-aPKC system: Lessons in polarity. J. Cell Sci. 2006, 119, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Hirose, T.; Tamai, Y.; Hirai, S.; Nagashima, Y.; Fujimoto, T.; Tabuse, Y.; Kemphues, K.J.; Ohno, S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J. Cell Biol. 1998, 143, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Mashukova, A.; Oriolo, A.S.; Wald, F.A.; Casanova, M.L.; Kroger, C.; Magin, T.M.; Omary, M.B.; Salas, P.J. Rescue of atypical protein kinase C in epithelia by the cytoskeleton and Hsp70 family chaperones. J. Cell Sci. 2009, 122, 2491–2503. [Google Scholar] [CrossRef] [PubMed]
- Wald, F.A.; Oriolo, A.S.; Casanova, M.L.; Salas, P.J. Intermediate filaments interact with dormant ezrin in intestinal epithelial cells. Mol. Biol. Cell 2005, 16, 4096–4107. [Google Scholar] [CrossRef] [PubMed]
- Wald, F.A.; Oriolo, A.S.; Mashukova, A.; Fregien, N.L.; Langshaw, A.H.; Salas, P.J. Atypical protein kinase C (iota) activates ezrin in the apical domain of intestinal epithelial cells. J. Cell Sci. 2008, 121, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Van Furden, D.; Johnson, K.; Segbert, C.; Bossinger, O. The C. elegans ezrin-radixin-moesin protein ERM-1 is necessary for apical junction remodelling and tubulogenesis in the intestine. Dev. Biol. 2004, 272, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.L.; Priess, J.R. A role for the centrosome and PAR-3 in the hand-off of MTOC function during epithelial polarization. Curr. Biol. 2012, 22, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, A.; Wehman, A.M.; Nance, J. PAR-3 mediates the initial clustering and apical localization of junction and polarity proteins during C. elegans intestinal epithelial cell polarization. Development 2010, 137, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Totong, R.; Achilleos, A.; Nance, J. PAR-6 is required for junction formation but not apicobasal polarization in C. elegans embryonic epithelial cells. Development 2007, 134, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Gloerich, M.; ten Klooster, J.P.; Vliem, M.J.; Koorman, T.; Zwartkruis, F.J.; Clevers, H.; Bos, J.L. Rap2A links intestinal cell polarity to brush border formation. Nat. Cell Biol. 2012, 14, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Pruss, R.M.; Mirsky, R.; Raff, M.C.; Thorpe, R.; Dowding, A.J.; Anderton, B.H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell 1981, 27, 419–428. [Google Scholar] [CrossRef]
- Oakley, B.R.; Oakley, C.E.; Yoon, Y.; Jung, M.K. γ-Tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell 1990, 61, 1289–1301. [Google Scholar] [CrossRef]
- Helfand, B.T.; Chang, L.; Goldman, R.D. The dynamic and motile properties of intermediate filaments. Annu. Rev. Cell Dev. Biol. 2003, 19, 445–467. [Google Scholar] [CrossRef] [PubMed]
- Oriolo, A.S.; Wald, F.A.; Ramsauer, V.P.; Salas, P.J. Intermediate filaments: A role in epithelial polarity. Exp. Cell Res. 2007, 313, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, K.; Sato, M.; Sato, K.; Nakajima-Shimada, J.; Harada, A. Caenorhabditis elegans chaperonin CCT/TRiC is required for actin and tubulin biogenesis and microvillus formation in intestinal epithelial cells. Mol. Biol. Cell 2014, 25, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Wayt, J.; Bretscher, A. Cordon bleu serves as a platform at the basal region of microvilli, where it regulates microvillar length through its WH2 domains. Mol. Biol. Cell 2014, 25, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Grega-Larson, N.E.; Crawley, S.W.; Erwin, A.L.; Tyska, M.J. Cordon bleu promotes the assembly of brush border microvilli. Mol. Biol. Cell 2015, 26, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Crawley, S.W.; Mooseker, M.S.; Tyska, M.J. Shaping the intestinal brush border. J. Cell Biol. 2014, 207, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Tilney, L.G.; Cardell, R.R. Factors controlling the reassembly of the microvillous border of the small intestine of the salamander. J. Cell Biol. 1970, 47, 408–422. [Google Scholar] [CrossRef] [PubMed]




| Intermediate Filament Protein | Distribution | RNAi Phenotype | Reference | |||
|---|---|---|---|---|---|---|
| Feeding | Microinjection | |||||
| 20 °C | 25 °C | 20 °C | 25 °C | |||
| IFB-2 | intestine | n.d. | n.d. | n.d. | n.d. | [36,37,41,43] |
| IFC-1 | intestine, hypodermis, pharyngeal junctions of early larvae | n.d. | early larval arrest (10%), abnormal epidermal morphology mostly in head region, occasional muscle detachment defects | n.d. | late embryonic lethal (7%), early larval arrest (16%), abnormal epidermal morphology mostly in head region, occasional muscle detachment defects | [36] |
| IFC-2 | intestine | adult lethal (3%) | adult lethal (10%), ruptured vulva/anus | dumpy (10%) | adult lethal (16%), ruptured vulva/anus | [36] |
| luminal invaginations into cytoplasm of intestinal cells | [37] | |||||
| IFD-1 | intestine | n.d. | n.d. | n.d. | n.d. | [36] |
| IFD-2 | intestine | n.d. | n.d. | n.d. | late embryonic lethal (10%), early larval arrest (3%), morphological defects | [36] |
| IFP-1 (formerly IFE-1) | intestine | n.d. | n.d. | n.d. | n.d. | [36] |
| Transgenesis | Intestinal Phenotype | Reference |
|---|---|---|
| KRT8−/− | colorectal hyperplasia | [20] |
| scattered γ-tubulin, disorganized microtubules, reduced apical syntaxin, aberrant intracellular localization of CFTR, sucrase isomaltase, alkaline phosphatase | [21] | |
| diarrhea, reduced apical F-actin, partial loss of H+, K+-ATPase and basolateral redistribution of the anion exchanger AE1/2 and the Na-transporter EnaC-γ | [49] | |
| spontaneous chronic T helper type 2 colitis | [50] | |
| microflora-dependent resistance to apoptosis | [51] | |
| KRT8+/− | increased crypt length, increased sensitivity to experimental colitis | [22] |
| KRT7−/− | n.d. | [52] |
| KRT18−/− | n.d. | [46] |
| KRT19−/− | n.d. | [45] |
| keratin type I−/− (except KRT18) | n.d. | [44] |
| hK18 R89C | partial disruption of keratin filament network | [48] |
| aggregate formation | [47] | |
| hK20 R80H | partial disruption of keratin filament network | [15] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coch, R.A.; Leube, R.E. Intermediate Filaments and Polarization in the Intestinal Epithelium. Cells 2016, 5, 32. https://doi.org/10.3390/cells5030032
Coch RA, Leube RE. Intermediate Filaments and Polarization in the Intestinal Epithelium. Cells. 2016; 5(3):32. https://doi.org/10.3390/cells5030032
Chicago/Turabian StyleCoch, Richard A., and Rudolf E. Leube. 2016. "Intermediate Filaments and Polarization in the Intestinal Epithelium" Cells 5, no. 3: 32. https://doi.org/10.3390/cells5030032
APA StyleCoch, R. A., & Leube, R. E. (2016). Intermediate Filaments and Polarization in the Intestinal Epithelium. Cells, 5(3), 32. https://doi.org/10.3390/cells5030032