Whole-Genome and Expression Analyses of Bamboo Aquaporin Genes Reveal Their Functions Involved in Maintaining Diurnal Water Balance in Bamboo Shoots
Abstract
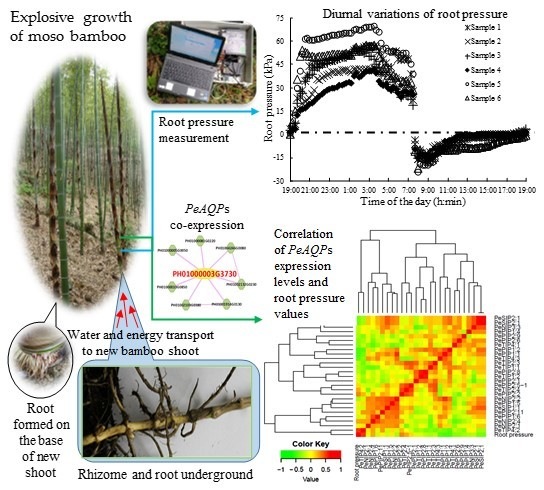
:1. Introduction
2. Materials and Methods
2.1. Identification and Structural Analysis of AQP Proteins and Sugar Transporters in Moso Bamboo
2.2. Phylogenetic Analysis of AQPs
2.3. Tissue-Specific Expression Pattern Analysis of Bamboo AQP Genes
2.4. Measurement of Root Pressure
2.5. Correlations and Network Analyses
2.6. Total RNA Isolation and qRT-PCR
2.7. In Situ Hybridization
3. Results
3.1. Identification and Classification of Bamboo Aquaporin Genes
3.2. Phylogenetic Analysis of AQPs
3.3. PeAQP Expression Pattern Analysis in Bamboo Tissues
3.4. Correlation Analysis of the Root Pressure and PeAQP Expression in Bamboo Shoots
3.5. Co-Expression Network Analyses of PeAQPs and Sugar Transport Genes
3.6. In Situ Hybridization of PeAQPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, B.Z.; Fu, M.Y.; Xie, J.Z.; Yang, X.S.; Li, Z.C. Ecological functions of bamboo forest: Research and application. J. For. Res. 2005, 16, 143–147. [Google Scholar]
- Peng, Z.; Lu, Y.; Li, L.; Zhao, Q.; Feng, Q.; Gao, Z.; Lu, H.; Hu, T.; Yao, N.; Liu, K.; et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat. Genet. 2013, 45, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Peng, C.; Zhou, G.; Gu, H.; Li, Q.; Zhang, C. Dynamic allocation and transfer of non-structural carbohydrates, a possible mechanism for the explosive growth of Moso bamboo (Phyllostachys heterocycla). Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, H.; Cai, D.; Gao, Y.; Zhang, H.; Wang, Y.; Lin, C.; Ma, L.; Gu, L. Comprehensive profiling of rhizome-associated alternative splicing and alternative polyadenylation in moso bamboo (Phyllostachys edulis). Plant J. 2017, 91, 684–699. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Walsh, M.; Fricke, W. Rapid changes in root hydraulic conductivity and aquaporin expression in rice (Oryza sativa L.) in response to shoot removal—Xylem tension as a possible signal. Ann. Bot. 2016, 118, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.L.; Wen, G.S.; Zhang, M.R.; Zhang, R.M.; Cai, X.F.; Zeng, Y.Y.; Li, H.J. Water potential with Phyllostachys edulis in its fast-growth periods. J. Zhejiang A & F Univ. 2015, 32, 722–728. [Google Scholar]
- Yang, S.J.; Zhang, Y.J.; Goldstein, G.; Sun, M.; Ma, R.Y.; Cao, K.F. Determinants of water circulation in a woody bamboo species: Afternoon use and night-time recharge of culm water storage. Tree Physiol. 2015, 35, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, L.; Lou, Y.; Zhao, H.; Gao, Z. Genome-wide identification and characterization of aquaporin gene family in moso bamboo (Phyllostachys edulis). Mol. Biol. Rep. 2016, 43, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.F.; Yang, S.J.; Zhang, Y.J.; Brodribb, T.J. The maximum height of grasses is determined by roots. Ecol. Lett. 2012, 15, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Mei, T.; Fang, D.; Roll, A.; Niu, F.; Hendrayanto; Holscher, D. Water use patterns of four tropical bamboo species assessed with sap flux measurements. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Yang, H.Q.; An, M.Y.; Gu, Z.J.; Tian, B. Genetic diversity and differentiation of dendrocalamus membranaceus (Poaceae: Bambusoideae), a declining bamboo species in Yunnan, China, as based on inter-simple sequence repeat (ISSR) analysis. Int. J. Mol. Sci. 2012, 13, 4446–4457. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed]
- Shekoofa, A.; Sinclair, T.R. Aquaporin activity to improve crop drought tolerance. Cells 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.B.; Sankararamakrishnan, R. Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: Characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, J.; Yu, J.W.; Park, S.W. Genome-wide analysis and expression profiling of the Solanum tuberosum aquaporins. Plant Physiol. Biochem. 2013, 73, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Zhong, X.; Li, B.; Wang, W.; Yue, Z.; Lei, J.; Guo, W.; Huang, X. Genome-wide identification and characterization of aquaporin genes (AQPs) in Chinese cabbage (Brassica rapa ssp. pekinensis). Mol. Genet. Genom. 2014, 289, 1131–1145. [Google Scholar] [CrossRef] [PubMed]
- Ariani, A.; Gepts, P. Genome-wide identification and characterization of aquaporin gene family in common bean (Phaseolus vulgaris L.). Mol. Genet. Genom. 2015, 290, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Johanson, U.; Karlsson, M.; Johansson, I.; Gustavsson, S.; Sjovall, S.; Fraysse, L.; Weig, A.R.; Kjellbom, P. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001, 126, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, L.; Lou, Y.; Zhao, H.; Yang, Y.; Wang, S.; Gao, Z. The bamboo aquaporin gene PeTIP4;1-1 confers drought and salinity tolerance in transgenic Arabidopsis. Plant Cell Rep. 2017, 36, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gao, Z.; Wang, L.; Wang, J.; Wang, S.; Fei, B.; Chen, C.; Shi, C.; Liu, X.; Zhang; et al. Chromosome-level reference genome and alternative splicing atlas of moso bamboo (Phyllostachys edulis). GigaScience 2018, 7. [Google Scholar] [CrossRef]
- Tajkhorshid, E.; Nollert, P.; Jensen, M.O.; Miercke, L.J.; O’Connell, J.; Stroud, R.M.; Schulten, K. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science 2002, 296, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Sankararamakrishnan, R. Homology modeling of major intrinsic proteins in rice, maize and Arabidopsis: Comparative analysis of transmembrane helix association and aromatic/arginine selectivity filters. BMC Struct. Biol. 2007, 7. [Google Scholar] [CrossRef] [PubMed]
- Mitani-Ueno, N.; Yamaji, N.; Zhao, F.J.; Ma, J.F. The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J. Exp. Bot. 2011, 62, 4391–4398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayadi, M.; Cavez, D.; Miled, N.; Chaumont, F.; Masmoudi, K. Identification and characterization of two plasma membrane aquaporins in durum wheat (Triticum turgidum L. subsp. durum) and their role in abiotic stress tolerance. Plant Physiol. Biochem. 2011, 49, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Andorf, C.M.; Cannon, E.K.; Portwood, J.L., 2nd.; Gardiner, J.M.; Harper, L.C.; Schaeffer, M.L.; Braun, B.L.; Campbell, D.A.; Vinnakota, A.G.; Sribalusu, V.V.; et al. MaizeGDB update: New tools, data and interface for the maize model organism database. Nucleic Acids Res. 2016, 44, D1195–D1201. [Google Scholar] [CrossRef] [PubMed]
- E, Z.G.; Wang, L. Construction and application of platform for rice scientific data. Chin. J. Rice Sci. 2015, 29, 653–657. [Google Scholar]
- Chaumont, F.; Barrieu, F.; Wojcik, E.; Chrispeels, M.J.; Jung, R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 2001, 125, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.X.; Moon, S.; Jung, K.H. Genome-wide expression analysis of rice aquaporin genes and development of a functional gene network mediated by aquaporin expression in roots. Planta 2013, 238, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, A.J. Java treeview—Extensible visualization of microarray data. Bioinformatics 2004, 20, 3246–3248. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Ma, J.; Guo, Q.; Li, X.; Wang, H.; Lu, M. Selection of reference genes for quantitative real-time PCR in bamboo (Phyllostachys edulis). PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Traas, J. Whole-mount in situ hybridization of RNA probes to plant tissues. CSH Protocols 2008. [CrossRef] [PubMed]
- Zhao, H.; Peng, Z.; Fei, B.; Li, L.; Hu, T.; Gao, Z.; Jiang, Z. BambooGDB: A bamboo genome database with functional annotation and an analysis platform. Database 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- McGaughey, S.A.; Osborn, H.L.; Chen, L.; Pegler, J.L.; Tyerman, S.D.; Furbank, R.T.; Byrt, C.S.; Grof, C.P. Roles of aquaporins in Setaria viridis stem development and sugar storage. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Liese, W. The Anatomy of Bamboo Culms; Brill: Leiden, The Netherlands, 1998; ISBN 8186247262. [Google Scholar]
- Chavarria, G.; Santos, H.P.D. Plant water relations: Absorption, transport and control mechanisms. Adv. Sel. Plant Physiol. Asp. 2012. [Google Scholar] [CrossRef]
- Henzler, T.; Steudle, E. Reversible closing of water channels in chara internodes provides evidence for a composite transport model of the plasma membrane. J. Exp. Bot. 1995, 46, 199–209. [Google Scholar] [CrossRef]
- Li, R.; Werger, M.J.; During, H.J.; Zhong, Z.C. Carbon and nutrient dynamics in relation to growth rhythm in the giant bamboo. Plant Soil 1998, 201, 113–123. [Google Scholar] [CrossRef]
- Callis, J.; Fromm, M.; Walbot, V. Introns increase gene expression in cultured maize cells. Gene Dev. 1987, 1, 1183–1200. [Google Scholar] [CrossRef] [PubMed]
- Brinster, R.L.; Allen, J.M.; Behringer, R.R.; Gelinas, R.E.; Palmiter, R.D. Introns increase transcriptional efficiency in transgenic mice. Proc. Natl. Acad. Sci. USA. 1988, 85, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Reinders, A.; Sivitz, A.B.; Starker, C.G.; Gantt, J.S.; Ward, J.M. Functional analysis of LjSUT4, a vacuolar sucrose transporter from Lotus japonicus. Plant Mol. Biol. 2008, 68, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Slewinski, T.L. Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: A physiological perspective. Mol. Plant 2011, 4, 641–662. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.J.; Byrt, C.S.; Patrick, J.W.; Grof, C.P. Are sucrose transporter expression profiles linked with patterns of biomass partitioning in Sorghum phenotypes? Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liese, W.; Köhl, M. (Eds.) Bamboo—The Plant and Its Uses; Springer International Publishing: Hamburg, Germany, 2015; ISBN 978-3-319-14132-9. [Google Scholar]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Wang, S.; Lou, Y.; Zhu, C.; Zhao, H.; Li, Y.; Li, X.; Gao, Z. Whole-Genome and Expression Analyses of Bamboo Aquaporin Genes Reveal Their Functions Involved in Maintaining Diurnal Water Balance in Bamboo Shoots. Cells 2018, 7, 195. https://doi.org/10.3390/cells7110195
Sun H, Wang S, Lou Y, Zhu C, Zhao H, Li Y, Li X, Gao Z. Whole-Genome and Expression Analyses of Bamboo Aquaporin Genes Reveal Their Functions Involved in Maintaining Diurnal Water Balance in Bamboo Shoots. Cells. 2018; 7(11):195. https://doi.org/10.3390/cells7110195
Chicago/Turabian StyleSun, Huayu, Sining Wang, Yongfeng Lou, Chenglei Zhu, Hansheng Zhao, Ying Li, Xueping Li, and Zhimin Gao. 2018. "Whole-Genome and Expression Analyses of Bamboo Aquaporin Genes Reveal Their Functions Involved in Maintaining Diurnal Water Balance in Bamboo Shoots" Cells 7, no. 11: 195. https://doi.org/10.3390/cells7110195
APA StyleSun, H., Wang, S., Lou, Y., Zhu, C., Zhao, H., Li, Y., Li, X., & Gao, Z. (2018). Whole-Genome and Expression Analyses of Bamboo Aquaporin Genes Reveal Their Functions Involved in Maintaining Diurnal Water Balance in Bamboo Shoots. Cells, 7(11), 195. https://doi.org/10.3390/cells7110195