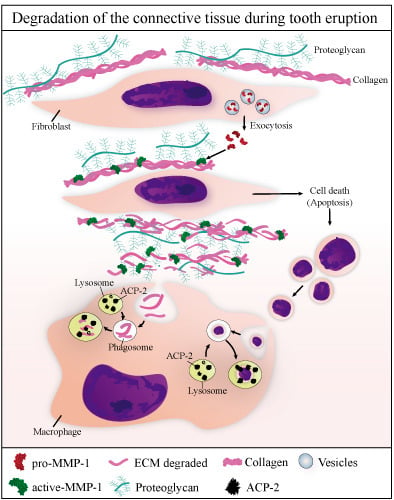
Matrix Metalloproteinase-1 and Acid Phosphatase in the Degradation of the Lamina Propria of Eruptive Pathway of Rat Molars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Light Microscopy
2.2. Collagen Content Measurement in the Eruptive Pathway
2.3. Immunohistochemical Detection of MMP-1
2.4. Numerical Density of MMP-1-Immunolabeled Cells
2.5. Immunohistochemical Detection of Acid Phosphatase (ACP-2)
2.6. Protein Extraction and Western Blot for MMP-1 and ACP-2
2.7. Transmission Electron Microscopy
2.8. Ultrastructural Localization of Acid Phosphatase Activity
2.9. Statistical Analysis
3. Results
3.1. Morphological Findings and Content of Birefringent Collagen
3.2. MMP-1 Immunoexpression in the Lamina Propria
3.3. ACP-2 Immunoexpression in the Lamina Propria
3.4. Detection of MMP-1 and ACP-2 by Western Blot
3.5. Ultrastructural Localization of Acid Phosphatase Activity
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurol, J. Impacted and ankylosed teeth: Why, when, and how to intervene. Am. J. Orthodont. Dent. Orthop. 2006, 129, S86–S90. [Google Scholar] [CrossRef] [PubMed]
- Loriato, L.B.; Machado, A.W.; Souki, B.Q.; Pereira, T.J. Late diagnosis of dentoalveolar ankylosis: Impact on effectiveness and efficiency of orthodontic treatment. Am. J. Orthodont. Dent. Orthop. 2009, 135, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Frazier-Bowers, S.A.; Puranik, C.P.; Mahaney, M.C. The etiology of eruption disorders—Further evidence of a ‘genetic paradigm’. Semin. Orthodont. 2010, 16, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Kreiborg, S.; Jensen, B.L. Tooth formation and eruption—Lessons learnt from cleidocranial dysplasia. Eur. J. Oral Sci. 2018, 126, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Arul, A.S.; Arul, A.S.; Chitra, S. Erupted complex odontoma of the posterior maxilla: A rarity. J. Nat. Sci. Biol. Med. 2015, 6, S167–S169. [Google Scholar] [PubMed]
- Marks, S.C., Jr.; Schroeder, H.E. Tooth eruption: Theories and facts. Anat. Rec. 1996, 245, 374–393. [Google Scholar] [CrossRef] [Green Version]
- Kjær, I. Mechanism of human tooth eruption: Review article including a new theory for future studies on the eruption process. Scientifica 2014, 2014, 341905. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P. Tooth eruption without roots. J. Dent. Res. 2013, 92, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Cerri, P.S.; Pereira-Júnior, J.A.; Biselli, N.B.; Sasso-Cerri, E. Mast cells and MMP-9 in the lamina propria during eruption of rat molars: Quantitative and immunohistochemical evaluation. J. Anat. 2010, 217, 116–125. [Google Scholar] [CrossRef] [PubMed]
- De Pizzol Júnior, J.P.; Sasso-Cerri, E.; Cerri, P.S. Apoptosis and reduced microvascular density of the lamina propria during tooth eruption in rats. J. Anat. 2015, 227, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.E. Cellular and molecular basis of tooth eruption. Orthodont. Craniofac. Res. 2009, 12, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yao, S.; Wise, G.E. MyD88 expression in the rat dental follicle: Implications for osteoclastogenesis and tooth eruption. Eur. J. Oral Sci. 2010, 118, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yao, S.; Wise, G.E. Regulation of SFRP-1 expression in the rat dental follicle. Connect. Tissue Res. 2012, 53, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, B.; Simon, Y.; Jacques, J.; Hess, E.; Choi, Y.W.; Blin-Wakkach, C.; Mueller, C.; Berdal, A.; Lézot, F. Bone resorption control of tooth eruption and root morphogenesis: Involvement of the receptor activator of NF-κB (RANK). J. Cell. Physiol. 2011, 226, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Golestani, R.; Razavian, M.; Ye, Y.; Zhang, J.; Jung, J.J.; Toczek, J.; Gona, K.; Kim, H.Y.; Elias, J.A.; Lee, C.G.; et al. Matrix metalloproteinase-targeted imaging of lung inflammation and remodeling. J. Nucl. Med. 2017, 58, 138–143. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.A.; de Pizzol-Júnior, J.P.; Longhini, R.; Sasso-Cerri, E.; Cerri, P.S. Cimetidine reduces interleukin-6, matrix metalloproteinases-1 and -9 immunoexpression in the gingival mucosa of rat Molars with induced periodontal disease. J. Periodontol. 2017, 88, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Aalinkeel, R.; Nair, B.B.; Reynolds, J.L.; Sykes, D.E.; Mahajan, S.D.; Chadha, K.C.; Schwartz, S.A. Overexpression of MMP-9 contributes to invasiveness of prostate cancer cell line LNCaP. Immunol. Invest. 2011, 40, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz-Zając, M.; Mroczko, B.; Słowik, A. Matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in amyotrophic lateral sclerosis (ALS). J. Neural Transm. 2014, 121, 1387–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincenti, M.P.; White, L.A.; Schroen, D.J.; Benbow, U.; Brinckerhoff, C.E. Regulating expression of the gene for matrix metalloproteinase-1 (collagenase): Mechanisms that control enzyme activity, transcription, and mRNA stability. Crit. Rev. Eukaryot. Gene Expr. 1996, 6, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Brinckerhoff, C.E.; Rutter, J.L.; Benbow, U. Interstitial collagenases as markers of tumor progression. Clin. Cancer Res. 2000, 6, 4823–4830. [Google Scholar] [PubMed]
- Bartlett, J.D.; Zhou, Z.; Skobe, Z.; Dobeck, J.M.; Tryggvason, K. Delayed tooth eruption in membrane type-1 matrix metalloproteinase deficient mice. Connect. Tissue Res. 2003, 44, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Kim, M.H.; Chae, C.H.; Jung, Y.K.; Choi, J.Y. Downregulation of matrix metalloproteinases in hyperplastic dental follicles results in abnormal tooth eruption. BMB Rep. 2008, 41, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Bull, H.; Murray, P.G.; Thomas, D.; Fraser, A.M.; Nelson, P.N. Acid phosphatases. Mol. Pathol. 2002, 55, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deporter, D.A.; Ten Cate, A.R. Fine structural localization of acid and alkaline phosphatase in collagen-containing vesicles of fibroblasts. J. Anat. 1973, 114, 457–461. [Google Scholar] [PubMed]
- Katz, S.G. Extracellular breakdown of collagen by mice decidual cells. A cytochemical and ultrastructural study. Biocell 2005, 29, 261–270. [Google Scholar] [PubMed]
- Cerri, P.S.; Freymüller, E.; Katchburian, E. Apoptosis in the early developing periodontium of rat molars. Anat. Rec. 2000, 258, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Moss, D.W.; Raymond, F.D.; Wile, D.B. Clinical and biological aspects of acid phosphatase. Crit. Rev. Clin. Lab. Sci. 1995, 32, 431–467. [Google Scholar] [CrossRef] [PubMed]
- Suter, A.; Everts, V.; Boyde, A.; Jones, S.J.; Lüllmann-Rauch, R.; Hartmann, D.; Hayman, A.R.; Cox, T.M.; Evans, M.J.; Meister, T.; et al. Overlapping functions of lysosomal acid phosphatase (LAP) and tartrate-resistant acid phosphatase (Acp5) revealed by doubly deficient mice. Development 2001, 128, 4899–4910. [Google Scholar] [PubMed]
- Bailey, K.; Balaei, M.R.; Mannan, A.; Del Bigio, M.R.; Marzban, H. Purkinje cell compartmentation in the cerebellum of the lysosomal Acid phosphatase 2 mutant mouse (nax-naked-ataxia mutant mouse). PLoS ONE 2014, 9, e94327. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Kremer, M.J. Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 2001, 7–15. [Google Scholar] [CrossRef]
- Marynka-Kalmani, K.; Treves, S.; Yafee, M.; Rachima, H.; Gafni, Y.; Cohen, M.A.; Pitaru, S. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells. 2010, 28, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Manni, M.L.; Czajka, C.A.; Oury, T.D.; Gilbert, T.W. Extracellular matrix powder protects against bleomycin-induced pulmonary fibrosis. Tissue Eng. Part A 2011, 17, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.; Whittaker, P. Collagen and picrosirius red staining: A polarized light assessment of fibrillar hue and spatial distribution. Braz. J. Morphol. Sci. 2005, 22, 97–104. [Google Scholar]
- Koshimizu, J.Y.; Beltrame, F.L.; de Pizzol, J.P., Jr.; Cerri, P.S.; Caneguim, B.H.; Sasso-Cerri, E. NF-kB overexpression and decreased immunoexpression of AR in the muscular layer is related to structural damages and apoptosis in cimetidine-treated rat vas deferens. Reprod. Biol. Endocrinol. 2013, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerri, P.S. Osteoblasts engulf apoptotic bodies during alveolar bone formation in the rat maxilla. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 286, 833–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barka, T. Electron histochemical localization of acid phosphatase activity in the small intestine of mouse. J. Histochem. Cytochem. 1964, 12, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilcher, B.K.; Dumin, J.A.; Sudbeck, B.D.; Krane, S.M.; Welgus, H.G.; Parks, W.C. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J. Cell Biol. 1997, 137, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Limb, G.A.; Matter, K.; Murphy, G.; Cambrey, A.D.; Bishop, P.N.; Morris, G.E.; Khaw, P.T. Matrix metalloproteinase-1 associates with intracellular organelles and confers resistance to lamin A/C degradation during apoptosis. Am. J. Pathol. 2005, 166, 1555–1563. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Nerlich, A.G.; Lichtinghagen, R.; Sommerhoff, C.P. Matrix metalloproteinases (MMPs) in breast cancer cell lines of different tumorigenicity. Anticancer Res. 2001, 21, 3821–3828. [Google Scholar] [PubMed]
- Inoue, T.; Yashiro, M.; Nishimura, S.; Maeda, K.; Sawada, T.; Ogawa, Y.; Sowa, M.; Chung, K.H. Matrix metalloproteinase-1 expression is a prognostic factor for patients with advanced gastric cancer. Int. J. Mol. Med. 1999, 4, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, G.; Biondi, M.L.; Mangoni, J.; Leviti, S.; DeMonti, M.; Guagnellini, E.; Scorza, R. Matrix metalloproteinase-1 promoter polymorphism 1G/2G is correlated with colorectal cancer invasiveness. Clin. Cancer Res. 2001, 7, 2344–2346. [Google Scholar] [PubMed]
- Nikkola, J.; Vihinen, P.; Vlaykova, T.; Hahka-Kemppinen, M.; Kähäri, V.M.; Pyrhönen, S. High expression levels of collagenase-1 and stromelysin-1 correlate with shorter disease-free survival in human metastatic melanoma. Int. J. Cancer 2002, 97, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Piérard, G.E. Sirius red polarization method is useful to visualize the organization of connective tissues but not the molecular composition of their fibrous polymers. Matrix 1989, 9, 68–71. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Montes, G.S.; Sanchez, E.M. The influence of tissue section thickness on the study of collagen by the picrosirius-polarization method. Histochemistry 1982, 74, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tamayo, R.; Montfort, I. The susceptibility of hepatic collagen to homologous collagenase in human and experimental cirrhosis of the liver. Am. J. Pathol. 1980, 100, 427–442. [Google Scholar] [PubMed]
- Amălinei, C.; Căruntu, I.D.; Giuşcă, S.E.; Bălan, R.A. Matrix metalloproteinases involvement in pathologic conditions. Rom. J. Morphol. Embryol. 2010, 51, 215–228. [Google Scholar] [PubMed]
- Butoi, E.; Gan, A.M.; Tucureanu, M.M.; Stan, D.; Macarie, R.D.; Constantinescu, C.; Calin, M.; Simionescu, M.; Manduteanu, I. Cross-talk between macrophages and smooth muscle cells impairs collagen and metalloprotease synthesis and promotes angiogenesis. Biochim. Biophys. Acta 2016, 1863, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Du, G.L.; Chen, W.Y.; Li, X.N.; He, R.; Feng, P.F. Induction of MMP-1 and -3 by cyclical mechanical stretch is mediated by IL-6 in cultured fibroblasts of keratoconus. Mol. Med. Rep. 2017, 15, 3885–3892. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Woessner, J.F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999, 30, 21491–21494. [Google Scholar] [CrossRef]
- Huang, H.; Wise, G.E. Delay of tooth eruption in null mice devoid of the type I IL-1R gene. Eur. J. Oral. Sci. 2000, 108, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Prpic, V.; Pan, F.; Wise, G.E. TNF-alpha upregulates expression of BMP-2 and BMP-3 genes in the rat dental follicle—Implications for tooth eruption. Connect. Tissue Res. 2010, 51, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Ida-Yonemochi, H.; Noda, T.; Shimokawa, H.; Saku, T. Disturbed tooth eruption in osteopetrotic (op/op) mice: Histopathogenesis of tooth malformation and odontomas. J. Oral Pathol. Med. 2002, 31, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Stetler-Stevenson, W.G. Matrix metalloproteinases in angiogenesis: A moving target for therapeutic intervention. J. Clin. Invest. 1999, 103, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Chesler, N.C.; Ku, D.N.; Galis, Z.S. Transmural pressure induces matrix-degrading activity in porcine arteries ex vivo. Am. J. Physiol. 1999, 277, H2002–H2009. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Johnson, C.; Godin, D.; Magid, R.; Shipley, J.M.; Senior, R.M; Ivan, E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ. Res. 2002, 91, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Suri, L.; Gagari, E.; Vastardis, H. Delayed tooth eruption: Pathogenesis, diagnosis, and treatment. A literature review. Am. J. Orthodont. Dent. Orthop. 2004, 126, 432–445. [Google Scholar] [CrossRef]
- Kirstein, B.; Chambers, T.J.; Fuller, K. Secretion of tartrate-resistant acid phosphatase by osteoclasts correlates with resorptive behavior. J. Cell. Biochem. 2006, 98, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Solberg, L.B.; Brorson, S.H.; Stordalen, G.A.; Baekkevold, E.S.; Andersson, G.; Reinholt, F.P. Increased tartrate-resistant acid phosphatase expression in osteoblasts and osteocytes in experimental osteoporosis in rats. Calcif. Tissue Int. 2014, 94, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.T.; Knight, M.B.; Harakas, N.K.; Wittwer, A.J.; Feder, J. Determination of the number of endothelial cells in culture using an acid phosphatase assay. Anal. Biochem. 1986, 152, 136–140. [Google Scholar] [CrossRef]
- Turner, R.R.; Beckstead, J.H.; Warnke, R.A.; Wood, G.S. Endothelial cell phenotypic diversity. In situ demonstration of immunologic and enzymatic heterogeneity that correlates with specific morphologic subtypes. Am. J. Clin. Pathol. 1987, 87, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.G. Demonstration of extracellular acid phosphatase activity in the involuting, antimesometrial decidua in fed and acutely fasted mice by combined cytochemistry and electron microscopy. Anat. Rec. 1998, 252, 1–7. [Google Scholar] [CrossRef]
- Faccioli, C.K.; Chedid, R.A.; Mori, R.H.; Amaral, A.C.; Franceschini-Vicentini, I.B.; Vicentini, C.A. Acid and alkaline phosphatase localization in the digestive tract mucosa of the Hemisorubim platyrhynchos. Acta Histochem. 2016, 118, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Ashtari, N.; Jiao, X.; Rahimi-Balaei, M.; Amiri, S.; Mehr, S.E.; Yeganeh, B.; Marzban, H. Lysosomal acid phosphatase biosynthesis and dysfunction: A mini review focused on lysosomal enzyme dysfunction in brain. Curr. Mol. Med. 2016, 16, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Geier, C.; Kreysing, J.; Boettcher, H.; Pohlmann, R.; von Figura, K. Localization of lysosomal acid phosphatase mRNA in mouse tissues. J. Histochem. Cytochem. 1992, 40, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Lemanski, L.F.; Aldoroty, R. Role of acid phosphatase in the breakdown of yolk platelets in developing amphibian embryos. J. Morphol. 1977, 153, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Hartmann, D.; Lüllmann-Rauch, R.; Wolff, J.; Evers, M.; Köster, A.; Hetman, M.; von Figura, K.; Peters, C. Mice deficient in lysosomal acid phosphatase develop lysosomal storage in the kidney and central nervous system. J. Biol. Chem. 1997, 272, 18628–18635. [Google Scholar] [CrossRef] [PubMed]
- Pipan, N.; Sterle, M. Cytochemical analysis of organelle degradation in phagosomes and apoptotic cells of the mucoid epithelium of mice. Histochemistry 1979, 59, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.J.; Anderson, T.J. Ultrastructural observations on cell death by apoptosis in the “resting” human breast. Virchows Arch. A Pathol. Anat. Histopathol. 1981, 393, 193–203. [Google Scholar] [CrossRef]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000, 14, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, N.; Werb, Z.; Bissell, M.J. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc. Natl. Acad. Sci. USA 1996, 93, 3509–3513. [Google Scholar] [CrossRef] [PubMed]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pizzol Júnior, J.P.; Sasso-Cerri, E.; Cerri, P.S. Matrix Metalloproteinase-1 and Acid Phosphatase in the Degradation of the Lamina Propria of Eruptive Pathway of Rat Molars. Cells 2018, 7, 206. https://doi.org/10.3390/cells7110206
De Pizzol Júnior JP, Sasso-Cerri E, Cerri PS. Matrix Metalloproteinase-1 and Acid Phosphatase in the Degradation of the Lamina Propria of Eruptive Pathway of Rat Molars. Cells. 2018; 7(11):206. https://doi.org/10.3390/cells7110206
Chicago/Turabian StyleDe Pizzol Júnior, José Paulo, Estela Sasso-Cerri, and Paulo Sérgio Cerri. 2018. "Matrix Metalloproteinase-1 and Acid Phosphatase in the Degradation of the Lamina Propria of Eruptive Pathway of Rat Molars" Cells 7, no. 11: 206. https://doi.org/10.3390/cells7110206
APA StyleDe Pizzol Júnior, J. P., Sasso-Cerri, E., & Cerri, P. S. (2018). Matrix Metalloproteinase-1 and Acid Phosphatase in the Degradation of the Lamina Propria of Eruptive Pathway of Rat Molars. Cells, 7(11), 206. https://doi.org/10.3390/cells7110206